A Protofilament-Protofilament Interface in the Structure of Mouse α-Synuclein Fibrils
- PMID: 29925018
- PMCID: PMC6026376
- DOI: 10.1016/j.bpj.2018.05.011
A Protofilament-Protofilament Interface in the Structure of Mouse α-Synuclein Fibrils
Abstract
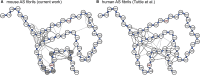
Fibrillar α-synuclein (AS) is the major component of Lewy bodies, the pathological hallmark of Parkinson's disease. Using solid-state nuclear magnetic resonance (ssNMR), we previously reported a structural characterization of mouse AS (mAS) fibrils and found that the secondary structure of the mAS fibrils is highly similar to a form of human AS (hAS) fibrils. Recently, a three-dimensional structure of these same hAS fibrils was determined by ssNMR and scanning transmission electron microscopy. Using medium- and long-range distance restraints obtained from ssNMR spectra, we found that the single protofilament structure of mAS fibrils is also similar to that of the hAS fibrils. However, residue-specific water accessibility of mAS fibrils probed by water polarization transfer ssNMR measurements indicates that residues S42-T44 and G84-V95 are largely protected from water even though they are located at the edge of the protofilament. Some of the corresponding resonances also exhibit peak doubling. These observations suggest that these residues may be involved in, to our knowledge, a novel protofilament-protofilament interface. We propose a structural model of mAS fibrils that incorporates this dimer interface.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

