Ionomycin-Induced Changes in Membrane Potential Alter Electroporation Outcomes in HL-60 Cells
- PMID: 29925024
- PMCID: PMC6026377
- DOI: 10.1016/j.bpj.2018.05.018
Ionomycin-Induced Changes in Membrane Potential Alter Electroporation Outcomes in HL-60 Cells
Abstract
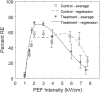
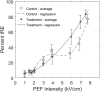
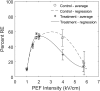
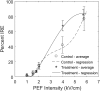
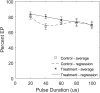
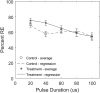
Previous studies have shown greater fluorophore uptake during electroporation on the anode-facing side of the cell than on the cathode-facing side. Based on these observations, we hypothesized that hyperpolarizing a cell before electroporation would decrease the requisite pulsed electric field intensity for electroporation outcomes, thereby yielding a higher probability of reversible electroporation at lower electric field strengths and a higher probability of irreversible electroporation (IRE) at higher electric field strengths. In this study, we tested this hypothesis by hyperpolarizing HL-60 cells using ionomycin before electroporation. These cells were then electroporated in a solution containing propidium iodide, a membrane integrity indicator. After 20 min, we added trypan blue to identify IRE cells. Our results showed that hyperpolarizing cells before electroporation alters the pulsed electric field intensity thresholds for reversible electroporation and IRE, allowing for greater control and selectivity of electroporation outcomes.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Electroporation of DC-3F cells is a dual process.Biophys J. 2015 Apr 7;108(7):1660-1671. doi: 10.1016/j.bpj.2015.01.038. Biophys J. 2015. PMID: 25863058 Free PMC article.
-
The Feasibility of Enhancing Susceptibility of Glioblastoma Cells to IRE Using a Calcium Adjuvant.Ann Biomed Eng. 2017 Nov;45(11):2535-2547. doi: 10.1007/s10439-017-1905-6. Epub 2017 Aug 28. Ann Biomed Eng. 2017. PMID: 28849278 Free PMC article.
-
Predicting electroporation of cells in an inhomogeneous electric field based on mathematical modeling and experimental CHO-cell permeabilization to propidium iodide determination.Bioelectrochemistry. 2014 Dec;100:52-61. doi: 10.1016/j.bioelechem.2014.03.011. Epub 2014 Mar 29. Bioelectrochemistry. 2014. PMID: 24731594
-
Induced transmembrane voltage and its correlation with electroporation-mediated molecular transport.J Membr Biol. 2010 Jul;236(1):3-13. doi: 10.1007/s00232-010-9279-9. Epub 2010 Jul 9. J Membr Biol. 2010. PMID: 20617432 Review.
-
Effect of electroporation on cardiac electrophysiology.Methods Mol Biol. 2008;423:433-48. doi: 10.1007/978-1-59745-194-9_34. Methods Mol Biol. 2008. PMID: 18370220 Review.
Cited by
-
A Note of Caution: Gramicidin Affects Signaling Pathways Independently of Its Effects on Plasma Membrane Conductance.Biomed Res Int. 2021 Oct 21;2021:2641068. doi: 10.1155/2021/2641068. eCollection 2021. Biomed Res Int. 2021. PMID: 34722759 Free PMC article.
References
-
- Weaver J.C. Electroporation: a general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 1993;51:426–435. - PubMed
-
- Abidor I.G., Arakelyan V.B., Tarasevich M.P. Electric breakdown of bilayer lipid membranes. I. The main experimental facts and their qualitative discussion. J. Electroanal. Chem. 1979;104:37–52.
-
- Weaver J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000;28:24–33.
-
- Neumann E., Sowers A.E., Jordan C.A. Plenum Press; New York: 1989. Electroporation and Electrofusion in Cell Biology.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

