Force Spectrum Microscopy Using Mitochondrial Fluctuations of Control and ATP-Depleted Cells
- PMID: 29925029
- PMCID: PMC6026348
- DOI: 10.1016/j.bpj.2018.05.002
Force Spectrum Microscopy Using Mitochondrial Fluctuations of Control and ATP-Depleted Cells
Abstract
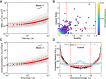
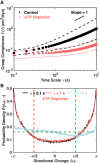
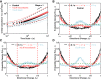
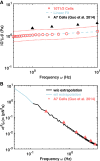
A single-cell assay of active and passive intracellular mechanical properties of mammalian cells could give significant insight into cellular processes. Force spectrum microscopy (FSM) is one such technique, which combines the spontaneous motion of probe particles and the mechanical properties of the cytoskeleton measured by active microrheology using optical tweezers to determine the force spectrum of the cytoskeleton. A simpler and noninvasive method to perform FSM would be very useful, enabling its widespread adoption. Here, we develop an alternative method of FSM using measurement of the fluctuating motion of mitochondria. Mitochondria of the C3H-10T1/2 cell line were labeled and tracked using confocal microscopy. Mitochondrial probes were selected based on morphological characteristics, and their mean-square displacement, creep compliance, and distributions of directional change were measured. We found that the creep compliance of mitochondria resembles that of particles in viscoelastic media. However, comparisons of creep compliance between controls and cells treated with pharmacological agents showed that perturbations to the actomysoin network had surprisingly small effects on mitochondrial fluctuations, whereas microtubule disruption and ATP depletion led to a significantly decreased creep compliance. We used properties of the distribution of directional change to identify a regime of thermally dominated fluctuations in ATP-depleted cells, allowing us to estimate the viscoelastic parameters for a range of timescales. We then determined the force spectrum by combining these viscoelastic properties with measurements of spontaneous fluctuations tracked in control cells. Comparisons with previous measurements made using FSM revealed an excellent match.
Copyright © 2018 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

