The Molecular Basis of G Protein-Coupled Receptor Activation
- PMID: 29925258
- PMCID: PMC6535337
- DOI: 10.1146/annurev-biochem-060614-033910
The Molecular Basis of G Protein-Coupled Receptor Activation
Abstract
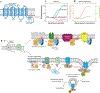
G protein-coupled receptors (GPCRs) mediate the majority of cellular responses to external stimuli. Upon activation by a ligand, the receptor binds to a partner heterotrimeric G protein and promotes exchange of GTP for GDP, leading to dissociation of the G protein into α and βγ subunits that mediate downstream signals. GPCRs can also activate distinct signaling pathways through arrestins. Active states of GPCRs form by small rearrangements of the ligand-binding, or orthosteric, site that are amplified into larger conformational changes. Molecular understanding of the allosteric coupling between ligand binding and G protein or arrestin interaction is emerging from structures of several GPCRs crystallized in inactive and active states, spectroscopic data, and computer simulations. The coupling is loose, rather than concerted, and agonist binding does not fully stabilize the receptor in an active conformation. Distinct intermediates whose populations are shifted by ligands of different efficacies underlie the complex pharmacology of GPCRs.
Keywords: 7-TM receptors; G protein–coupled receptor; GPCR; allostery; energy landscape; β2-adrenergic receptor.
Figures


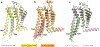
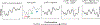
References
-
- Maguire ME, Van Arsdale PM, Glilman AG. 1976. An agonist-specific effect of guanine nucleotides on binding to the beta adrenergic receptor. Mol. Pharmacol 12:332–39 - PubMed
-
- De Lean A, Stadel JM, Lefkowitz RJ. 1980. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem 255:7108–17 - PubMed
-
- Henzler-Wildman K, Kern D. 2007. Dynamic personalities of proteins. Nature 450:964–72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

