Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways
- PMID: 29925372
- PMCID: PMC6011599
- DOI: 10.1186/s12917-018-1508-y
Selenium ameliorates Staphylococcus aureus-induced inflammation in bovine mammary epithelial cells by inhibiting activation of TLR2, NF-κB and MAPK signaling pathways
Abstract
Background: Staphylococcus aureus (S. aureus) internalization into bovine mammary epithelial cells (bMECs) is considered an important pathogenic mechanism for the establishment of mastitis. Given the interesting link between selenium (Se) status and mastitis, our objective was to prove that Se was essential to suppress pro-inflammatory mediators, in part, by modulation of Toll-like receptor2 (TLR2), nuclear factor kappaB (NF-κB) and mitogen activated protein kinase (MAPK) signal transduction pathway in bMECs.
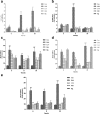
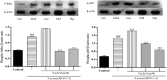
Results: Results showed that Se (0~ 16 μM) did not affect the growth of bMECs. The mRNA expression of TLR2, Myeloid differentiation factor 88 (Myd88), Interleukin-1 receptor-associated kinase4 (Irak4), Interleukin-1 receptor-associated kinase1 (Irak1) and TNF receptor-associated factor6 (Traf6) in TLR2 signal pathway were increased or significantly increased by S. aureus. Se played an important role in regulating the genes expression of TLR2, Myd88, Traf6 but not in controlling the expression of Irak4 and Irak1. In addition, Se exerted strong inhibitory effects on the genes expression of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) induced by S. aureus. To further investigate the possible signaling mechanisms involved in the processes, we analyzed the role of MAPK and NF-κB signaling pathway in inflammation response in S. aureus-stimulated bMECs in vitro. Results showed that the phosphorylation of inhibitory kappaB alpha (IκBα), p65, p38 and extracellular regulated protein kinase (Erk) were significantly increased in S. aureus-stimulated bMECs. It indicated that S. aureus activated NF-κB and MAPK signaling pathway. We also examined the effects of Se on the phosphorylation of IκBα, p65, p38 and Erk in NF-κB and MAPK signaling pathway, which have well been proved to control the synthesis and release of pro-inflammatory mediators during inflammation. The findings are exciting, that pretreatment with Se (4, 8 μM) significantly suppressed the phosphorylation of IκBα, p65, p38 and Erk.
Conclusions: These results suggest that Se down-regulates inflammatory mediators TNF-α, IL-1β and IL-6 gene expressions via TLR2, NF-κB and MAPK signaling pathway in S. aureus-stimulated bMECs, which may be responsible for the anti-inflammatory effect of Se.
Keywords: MAPK; Mammary; NF-κB; Se; TLR2.
Conflict of interest statement
Ethics approval
The protocol was approved by the Animal Care and Ethics Committee of Yangzhou University.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Polydatin ameliorates Staphylococcus aureus-induced mastitis in mice via inhibiting TLR2-mediated activation of the p38 MAPK/NF-κB pathway.Acta Pharmacol Sin. 2017 Feb;38(2):211-222. doi: 10.1038/aps.2016.123. Epub 2016 Nov 28. Acta Pharmacol Sin. 2017. PMID: 27890916 Free PMC article.
-
Taraxacum mongolicum protects against Staphylococcus aureus-infected mastitis by exerting anti-inflammatory role via TLR2-NF-κB/MAPKs pathways in mice.J Ethnopharmacol. 2021 Mar 25;268:113595. doi: 10.1016/j.jep.2020.113595. Epub 2020 Nov 16. J Ethnopharmacol. 2021. PMID: 33212175
-
Hederacoside-C Inhibition of Staphylococcus aureus-Induced Mastitis via TLR2 & TLR4 and Their Downstream Signaling NF-κB and MAPKs Pathways In Vivo and In Vitro.Inflammation. 2020 Apr;43(2):579-594. doi: 10.1007/s10753-019-01139-2. Inflammation. 2020. PMID: 31845052
-
Bioactive Compounds and Probiotics Mitigate Mastitis by Targeting NF-κB Signaling Pathway.Biomolecules. 2024 Aug 15;14(8):1011. doi: 10.3390/biom14081011. Biomolecules. 2024. PMID: 39199398 Free PMC article. Review.
-
Effects of Toll-like receptor 1 and 2 agonist Pam3CSK4 on uveal melanocytes and relevant experimental mouse model.Exp Eye Res. 2024 Feb;239:109749. doi: 10.1016/j.exer.2023.109749. Epub 2023 Dec 17. Exp Eye Res. 2024. PMID: 38113956 Review.
Cited by
-
Overview of Research Development on the Role of NF-κB Signaling in Mastitis.Animals (Basel). 2020 Sep 10;10(9):1625. doi: 10.3390/ani10091625. Animals (Basel). 2020. PMID: 32927884 Free PMC article. Review.
-
Effect of Usnea longissima ethyl acetate extract on acute oxidative and inflammatory lung damage from Staphylococcus aureus infection in rats.J Appl Biomed. 2023 Dec;21(4):200-207. doi: 10.32725/jab.2023.022. Epub 2023 Dec 7. J Appl Biomed. 2023. PMID: 38112459
-
Genetic polymorphisms in immune- and inflammation-associated genes and their association with bovine mastitis resistance/susceptibility.Front Immunol. 2023 Feb 23;14:1082144. doi: 10.3389/fimmu.2023.1082144. eCollection 2023. Front Immunol. 2023. PMID: 36911690 Free PMC article. Review.
-
Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period.Antioxidants (Basel). 2022 Mar 29;11(4):657. doi: 10.3390/antiox11040657. Antioxidants (Basel). 2022. PMID: 35453342 Free PMC article. Review.
-
Novel organic selenium source hydroxy-selenomethionine counteracts the blood-milk barrier disruption and inflammatory response of mice under heat stress.Front Immunol. 2022 Dec 1;13:1054128. doi: 10.3389/fimmu.2022.1054128. eCollection 2022. Front Immunol. 2022. PMID: 36532046 Free PMC article.
References
-
- Günther J, Liu S, Esch K, Schuberth HJ, Seyfert HM. Stimulated expression of TNF-α and IL-8, but not of lingual antimicrobial peptide reflects the concentration of pathogens contacting bovine mammary epithelial cells. Vet Immunol Immunopathol. 2010;135:152–157. doi: 10.1016/j.vetimm.2009.11.004. - DOI - PubMed
-
- Günther J, Kathrin E, Seyfert HM. Comparative kinetics of Escherichia coli and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S.aureus elicits a delayed response dominated by Interleukin-6 (IL-6) but not by IL-1α or tumor necrosis factor alpha. Infect Immun. 2011;79:695–707. doi: 10.1128/IAI.01071-10. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

