Indacaterol/glycopyrronium versus salmeterol/fluticasone in the prevention of clinically important deterioration in COPD: results from the FLAME study
- PMID: 29925383
- PMCID: PMC6011394
- DOI: 10.1186/s12931-018-0830-z
Indacaterol/glycopyrronium versus salmeterol/fluticasone in the prevention of clinically important deterioration in COPD: results from the FLAME study
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is a progressive disease and a composite endpoint could be an indicator of treatment effect on disease worsening. This post-hoc analysis assessed whether indacaterol/glycopyrronium (IND/GLY) 110/50 μg once daily reduced the risk of clinically important deterioration (CID) versus salmeterol/fluticasone (SFC) 50/500 μg twice daily in moderate-to-very severe COPD patients from the FLAME study.
Methods: CID was defined as ≥100 mL decrease in forced expiratory volume in 1 s (FEV1) or ≥ 4-unit increase in St. George's Respiratory Questionnaire (SGRQ) total score or a moderate-to-severe COPD exacerbation. Changes from baseline in the rate of moderate and severe exacerbations, time to first moderate-to-severe exacerbation, and change from baseline in the SGRQ score, measured after Week 12 up to Week 52, were assessed by presence of early CID (CID+) or absence of CID (CID-) at Week 12.
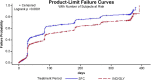
Results: IND/GLY significantly delayed the time to CID (hazard ratio [HR] (95% confidence interval [CI]), 0.72 [0.67-0.78]; P < 0.0001), and reduced the incidences of CID versus SFC. Additionally, IND/GLY delayed the time to CID in all patient subgroups. After 12 weeks until 52 weeks, CID+ patients had a significantly higher rate of moderate-to-severe exacerbations versus CID- patients (P < 0.0001); moreover, CID+ patients experienced moderate-to-severe exacerbations significantly earlier versus CID- patients (P < 0.0001). CID+ patients had a comparable change in the SGRQ total score versus CID- patients.
Conclusions: IND/GLY reduced the risk of CID versus SFC. CID had a significant impact on long-term exacerbation outcomes in patients with moderate-to-very severe COPD and a history of ≥1 exacerbations in the previous year.
Trial registration: Clinicaltrials.gov NCT01782326 .
Keywords: CID; COPD; Chronic obstructive pulmonary disease; Clinically important deterioration; FLAME; Indacaterol/glycopyrronium; LABA/LAMA; Salmeterol/fluticasone.
Conflict of interest statement
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki and the International Conference on Harmonisation and Good Clinical Practice guidelines. The protocol was approved by the regulatory authority for each country (where applicable) and an independent ethics committee at each center. All patients provided written informed consent.
Consent for publication
Not applicable.
Competing interests
AA has received honoraria for consultant services and speaking from Novartis, AstraZeneca, Boehringer Ingelheim, Sunovion Pharma, and GlaxoSmithKline. JAW has received no honoraria for lectures, consultant services and/or advisory boards. She has received research grants from Novartis, AstraZeneca, Boehringer Ingelheim, Johnson and Johnson, Sunovion Pharma, GlaxoSmithKline. KK is an employee and shareholder at Novartis Pharma AG. KK has previously received honoraria for speeches and consulting services from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN and Takeda, outside the submitted work. None of the authors received any compensation for the development of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) And prevention of chronic obstructive pulmonary disease. 2018. Global strategy for the diagnosis, management. - PubMed
-
- Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, D'Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. - DOI - PubMed
-
- Vogelmeier CF, Gaga M, Aalamian-Mattheis M, Greulich T, Marin JM, Castellani W, Ninane V, Lane S, Nunez X, Patalano F, et al. Efficacy and safety of direct switch to indacaterol/glycopyrronium in patients with moderate COPD: the CRYSTAL open-label randomised trial. Respir Res. 2017;18:140. doi: 10.1186/s12931-017-0622-x. - DOI - PMC - PubMed
-
- Anzueto AR, Vogelmeier CF, Kostikas K, Mezzi K, Fucile S, Bader G, Shen S, Banerji D, Fogel R. The effect of indacaterol/glycopyrronium versus tiotropium or salmeterol/fluticasone on the prevention of clinically important deterioration in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1325–1337. doi: 10.2147/COPD.S133307. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

