Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy
- PMID: 29925402
- PMCID: PMC6011351
- DOI: 10.1186/s13045-018-0624-2
Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy
Abstract
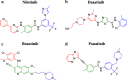
Bcr-Abl inhibitors paved the way of targeted therapy epoch. Imatinib was the first tyrosine kinase inhibitor to be discovered with high specificity for Bcr-Abl protein resulting from t(9, 22)-derived Philadelphia chromosome. Although the specific targeting of that oncoprotein, several Bcr-Abl-dependent and Bcr-Abl-independent mechanisms of resistance to imatinib arose after becoming first-line therapy in chronic myelogenous leukemia (CML) treatment.Consequently, new specific drugs, namely dasatinib, nilotinib, bosutinib, and ponatinib, were rationally designed and approved for clinic to override resistances. Imatinib fine mechanisms of action had been elucidated to rationally develop those second- and third-generation inhibitors. Crystallographic and structure-activity relationship analysis, jointly to clinical data, were pivotal to shed light on this topic. More recently, preclinical evidence on bafetinib, rebastinib, tozasertib, danusertib, HG-7-85-01, GNF-2, and 1,3,4-thiadiazole derivatives lay promising foundations for better inhibitors to be approved for clinic in the near future.Notably, structural mechanisms of action and drug design exemplified by Bcr-Abl inhibitors have broad relevance to both break through resistances in CML treatment and develop inhibitors against other kinases as targeted chemotherapeutics.
Keywords: Bcr-Abl; Bosutinib; Dasatinib; Imatinib; Leukemia; Nilotinib; Ponatinib; Structure-activity relationship; Targeted therapy; Tyrosine kinase inhibitors (TKIs).
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Zimmermann J, Buchdunger E, Mett H, Meyer T, Lydon NB. Potent and selective inhibitors of the Abl-kinase: phenylaminopyrimidine (PAP) derivatives. Bioorganic Med Chem Lett. 1997;7:187–192. doi: 10.1016/S0960-894X(96)00601-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

