S-amlodipine plus chlorthalidone vs. S-amlodipine plus telmisartan in hypertensive patients unresponsive to amlodipine monotherapy: study protocol for a randomized controlled trial
- PMID: 29925421
- PMCID: PMC6011241
- DOI: 10.1186/s13063-018-2636-1
S-amlodipine plus chlorthalidone vs. S-amlodipine plus telmisartan in hypertensive patients unresponsive to amlodipine monotherapy: study protocol for a randomized controlled trial
Abstract
Background: The efficacy of a combination of a calcium channel blocker (CCB) plus chlorthalidone (diuretic) versus a CCB plus an angiotensin receptor blocker (ARB) in patients not responding to CCB monotherapy has not been evaluated previously. We plan to compare the efficacy and safety of S-amlodipine (CCB) plus chlorthalidone versus S-amlodipine plus telmisartan (ARB) combinations among hypertension patients unresponsive to amlodipine monotherapy.
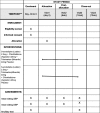
Methods/design: This study is a prospective, randomized, double-blind, multicenter, parallel, non-inferiority phase 4 study. Hypertension patients who have been treated with amlodipine (5 mg) or S-amlodipine (2.5 mg) monotherapy for ≥2 weeks and whose mean diastolic blood pressure (DBP) is greater than 90 mmHg will be randomized to either S-amlodipine (2.5 mg) plus chlorthalidone (25 mg) or S-amlodipine (2.5 mg) plus telmisartan (40 mg) therapy. The primary efficacy endpoint is mean sitting DBP change after 12 weeks of treatment. The study objective is to prove the non-inferiority of the former combination (test drug) as compared to the latter one (control) with a non-inferiority margin of 3 mmHg in mean DBP change. The secondary endpoints are 6-week DBP change, 6- and 12-week sitting systolic BP (SBP) change, and the attainment of the target BP (SBP < 140 mmHg or DBP < 90 mmHg). Urine albumin, albumin/creatinine ratio (ACR), pulse wave velocity, central BP, 24-h ambulatory BP monitoring, and body fluid composition analysis will be performed at each hospital's discretion. The sample size was estimated as 170 in total with 1:1 randomization.
Discussion: This is the first study comparing the efficacy of a CCB plus chlorthalidone versus a CCB plus an ARB in patients who are not responding to CCB single therapy. The study result will help clinicians to choose between chlorthalidone and telmisartan in CCB-unresponsive patients.
Trial registration: ClinicalTrials.gov , NCT03226340. Registered on 2 December 2015.
Keywords: Angiotensin receptor blocker; Calcium channel blocker; Combination; Hypertension.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by each institutional review board: Hallym University Sacred Heart Hospital (approval number 2015-I110), Samsung Medical Center (approval number 2015-07-097), Korea University Guro Hospital (approval number KUGH 15145-001), KyungHee University Hospital (approval number KMC IRB 1527-05), Seoul National University Hospital (approval number, 1507-059-687), Asan Medical Center(approval number S2015-1140-0001), Hanyang University Hospital (approval number 2015-07-015), Ajou University School of Medicine (approval number MED-CT4-15-209), Kangdong Sacred Heart Hospital (approval number 2015-07-012), and Seoul National University Bundang Hospital (approval number B-1507/307-005), and is being carried out in compliance with the Declaration of Helsinki.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Efficacy and safety of two fixed-dose combinations of S-amlodipine and telmisartan (CKD-828) versus S-amlodipine monotherapy in patients with hypertension inadequately controlled using S-amlodipine monotherapy: an 8-week, multicenter, randomized, double-blind, Phase III clinical study.Drug Des Devel Ther. 2016 Nov 23;10:3817-3826. doi: 10.2147/DDDT.S116847. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27920497 Free PMC article. Clinical Trial.
-
Combination of amlodipine plus angiotensin receptor blocker or diuretics in high-risk hypertensive patients: a 96-week efficacy and safety study.Am J Cardiovasc Drugs. 2012 Apr 1;12(2):137-42. doi: 10.2165/11598110-000000000-00000. Am J Cardiovasc Drugs. 2012. PMID: 22329591 Clinical Trial.
-
Telmisartan plus amlodipine in patients with moderate or severe hypertension: results from a subgroup analysis of a randomized, placebo-controlled, parallel-group, 4 x 4 factorial study.Postgrad Med. 2009 Mar;121(2):5-14. doi: 10.3810/pgm.2009.03.1972. Postgrad Med. 2009. PMID: 19332958 Clinical Trial.
-
Review: a single-pill combination of telmisartan plus amlodipine for the treatment of hypertension.Postgrad Med. 2011 Nov;123(6):58-65. doi: 10.3810/pgm.2011.11.2495. Postgrad Med. 2011. PMID: 22104454 Review.
-
A review of the benefits of early treatment initiation with single-pill combinations of telmisartan with amlodipine or hydrochlorothiazide.Vasc Health Risk Manag. 2013;9:521-8. doi: 10.2147/VHRM.S48291. Epub 2013 Sep 16. Vasc Health Risk Manag. 2013. PMID: 24082785 Free PMC article. Review.
References
-
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical