Octopamine signaling in the metazoan pathogen Schistosoma mansoni: localization, small-molecule screening and opportunities for drug development
- PMID: 29925529
- PMCID: PMC6078403
- DOI: 10.1242/dmm.033563
Octopamine signaling in the metazoan pathogen Schistosoma mansoni: localization, small-molecule screening and opportunities for drug development
Abstract
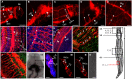
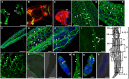
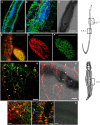
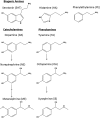
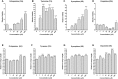
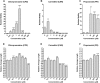
Schistosomiasis is a tropical disease caused by a flatworm trematode parasite that infects over 200 million people worldwide. Treatment and control of the disease rely on just one drug, praziquantel. The possibility of drug resistance coupled with praziquantel's variable efficacy encourages the identification of new drugs and drug targets. Disruption of neuromuscular homeostasis in parasitic worms is a validated strategy for drug development. In schistosomes, however, much remains to be understood about the organization of the nervous system, its component neurotransmitters and potential for drug discovery. Using synapsin as a neuronal marker, we map the central and peripheral nervous systems in the Schistosoma mansoni adult and schistosomulum (post-infective larva). We discover the widespread presence of octopamine (OA), a tyrosine-derived and invertebrate-specific neurotransmitter involved in neuromuscular coordination. OA labeling facilitated the discovery of two pairs of ganglia in the brain of the adult schistosome, rather than the one pair thus far reported for this and other trematodes. In quantitative phenotypic assays, OA and the structurally related tyrosine-derived phenolamine and catecholamine neurotransmitters differentially modulated schistosomulum motility and length. Similarly, from a screen of 28 drug agonists and antagonists of tyrosine-derivative signaling, certain drugs that act on OA and dopamine receptors induced robust and sometimes complex concentration-dependent effects on schistosome motility and length; in some cases, these effects occurred at concentrations achievable in vivo The present data advance our knowledge of the organization of the nervous system in this globally important pathogen and identify a number of drugs that interfere with tyrosine-derivative signaling, one or more of which might provide the basis for a new chemotherapeutic approach to treat schistosomiasis.This article has an associated First Person interview with the first author of the paper.
Keywords: Biogenic amine; Dopamine; Drug discovery; Nervous system; Neuromuscular; Octopamine; Schistosoma mansoni; Synapsin.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
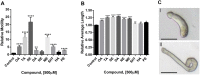
References
-
- Abdulla M.-H., Ruelas D. S., Wolff B., Snedecor J., Lim K.-C., Xu F., Renslo A. R., Williams J., McKerrow J. H. and Caffrey C. R. (2009). Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl. Trop. Dis. 3, e478 10.1371/journal.pntd.0000478 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

