Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice
- PMID: 29925537
- PMCID: PMC6176989
- DOI: 10.1242/dmm.032698
Pgc-1α repression and high-fat diet induce age-related macular degeneration-like phenotypes in mice
Abstract
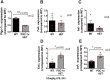
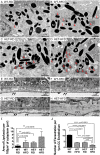
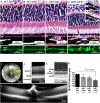
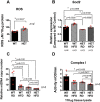
Age-related macular degeneration (AMD) is the major cause of blindness in the elderly in developed countries and its prevalence is increasing with the aging population. AMD initially affects the retinal pigment epithelium (RPE) and gradually leads to secondary photoreceptor degeneration. Recent studies have associated mitochondrial damage with AMD, and we have observed mitochondrial and autophagic dysfunction and repressed peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α; also known as Ppargc1a) in native RPE from AMD donor eyes and their respective induced pluripotent stem cell-derived RPE. To further investigate the effect of PGC-1α repression, we have established a mouse model by feeding Pgc-1α+/- mice with a high-fat diet (HFD) and investigated RPE and retinal health. We show that when mice expressing lower levels of Pgc-1α are exposed to HFD, they present AMD-like abnormalities in RPE and retinal morphology and function. These abnormalities include basal laminar deposits, thickening of Bruch's membrane with drusen marker-containing deposits, RPE and photoreceptor degeneration, decreased mitochondrial activity, increased levels of reactive oxygen species, decreased autophagy dynamics/flux, and increased inflammatory response in the RPE and retina. Our study shows that Pgc-1α is important in outer retina biology and that Pgc-1α+/- mice fed with HFD provide a promising model to study AMD, opening doors for novel treatment strategies.
Keywords: AMD; Autophagy; High-fat diet; Mitochondria; PGC-1α; RPE; Retinal degeneration.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


Similar articles
-
Potential of Telomerase in Age-Related Macular Degeneration-Involvement of Senescence, DNA Damage Response and Autophagy and a Key Role of PGC-1α.Int J Mol Sci. 2021 Jul 3;22(13):7194. doi: 10.3390/ijms22137194. Int J Mol Sci. 2021. PMID: 34281248 Free PMC article. Review.
-
PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium.Cell Death Dis. 2024 Jun 1;15(6):385. doi: 10.1038/s41419-024-06762-y. Cell Death Dis. 2024. PMID: 38824126 Free PMC article.
-
Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration.Redox Biol. 2019 Jan;20:1-12. doi: 10.1016/j.redox.2018.09.011. Epub 2018 Sep 14. Redox Biol. 2019. PMID: 30253279 Free PMC article.
-
Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration.J Transl Med. 2016 Dec 20;14(1):344. doi: 10.1186/s12967-016-1101-8. J Transl Med. 2016. PMID: 27998274 Free PMC article.
-
PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis.Int J Mol Sci. 2018 Aug 7;19(8):2317. doi: 10.3390/ijms19082317. Int J Mol Sci. 2018. PMID: 30087287 Free PMC article. Review.
Cited by
-
Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging.Oxid Med Cell Longev. 2019 Nov 4;2019:9825061. doi: 10.1155/2019/9825061. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31781358 Free PMC article. Review.
-
Potential of Telomerase in Age-Related Macular Degeneration-Involvement of Senescence, DNA Damage Response and Autophagy and a Key Role of PGC-1α.Int J Mol Sci. 2021 Jul 3;22(13):7194. doi: 10.3390/ijms22137194. Int J Mol Sci. 2021. PMID: 34281248 Free PMC article. Review.
-
Dysfunctional Autophagy, Proteostasis, and Mitochondria as a Prelude to Age-Related Macular Degeneration.Int J Mol Sci. 2023 May 15;24(10):8763. doi: 10.3390/ijms24108763. Int J Mol Sci. 2023. PMID: 37240109 Free PMC article. Review.
-
PGC-1α regulates the interplay between oxidative stress, senescence and autophagy in the ageing retina important in age-related macular degeneration.J Cell Mol Med. 2024 Apr;28(8):e18051. doi: 10.1111/jcmm.18051. J Cell Mol Med. 2024. PMID: 38571282 Free PMC article.
-
Autophagy in Age-Related Macular Degeneration: A Regulatory Mechanism of Oxidative Stress.Oxid Med Cell Longev. 2020 Aug 8;2020:2896036. doi: 10.1155/2020/2896036. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32831993 Free PMC article. Review.
References
-
- Ach T., Tolstik E., Messinger J. D., Zarubina A. V., Heintzmann R. and Curcio C. A. (2015). Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 56, 3242-3252. 10.1167/iovs.14-16274 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

