The impact of traditional neuroimaging methods on the spatial localization of cortical areas
- PMID: 29925602
- PMCID: PMC6142239
- DOI: 10.1073/pnas.1801582115
The impact of traditional neuroimaging methods on the spatial localization of cortical areas
Abstract
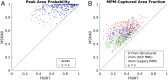
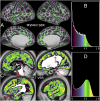
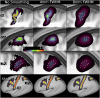
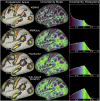
Localizing human brain functions is a long-standing goal in systems neuroscience. Toward this goal, neuroimaging studies have traditionally used volume-based smoothing, registered data to volume-based standard spaces, and reported results relative to volume-based parcellations. A novel 360-area surface-based cortical parcellation was recently generated using multimodal data from the Human Connectome Project, and a volume-based version of this parcellation has frequently been requested for use with traditional volume-based analyses. However, given the major methodological differences between traditional volumetric and Human Connectome Project-style processing, the utility and interpretability of such an altered parcellation must first be established. By starting from automatically generated individual-subject parcellations and processing them with different methodological approaches, we show that traditional processing steps, especially volume-based smoothing and registration, substantially degrade cortical area localization compared with surface-based approaches. We also show that surface-based registration using features closely tied to cortical areas, rather than to folding patterns alone, improves the alignment of areas, and that the benefits of high-resolution acquisitions are largely unexploited by traditional volume-based methods. Quantitatively, we show that the most common version of the traditional approach has spatial localization that is only 35% as good as the best surface-based method as assessed using two objective measures (peak areal probabilities and "captured area fraction" for maximum probability maps). Finally, we demonstrate that substantial challenges exist when attempting to accurately represent volume-based group analysis results on the surface, which has important implications for the interpretability of studies, both past and future, that use these volume-based methods.
Keywords: CIFTI grayordinates; blurring; cross-subject alignment; neuroimaging analysis; standard space.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Geschwind N. Selected Papers on Language and the Brain. Springer; Dordrecht, TheNetherlands: 1974. Disconnexion syndromes in animals and man; pp. 105–236.
-
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Johann Ambrosius Barth; Leipzig, Germany: 1909.
-
- Nieuwenhuys R. The myeloarchitectonic studies on the human cerebral cortex of the Vogt-Vogt school, and their significance for the interpretation of functional neuroimaging data. Brain Struct Funct. 2013;218:303–352. - PubMed
-
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. - PubMed
-
- Belliveau JW, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

