Optogenetic Inhibition of CGRPα Sensory Neurons Reveals Their Distinct Roles in Neuropathic and Incisional Pain
- PMID: 29925650
- PMCID: PMC6010565
- DOI: 10.1523/JNEUROSCI.3565-17.2018
Optogenetic Inhibition of CGRPα Sensory Neurons Reveals Their Distinct Roles in Neuropathic and Incisional Pain
Abstract
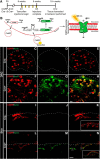
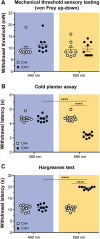
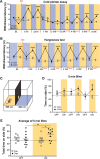
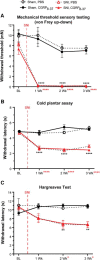
Cutaneous somatosensory neurons convey innocuous and noxious mechanical, thermal, and chemical stimuli from peripheral tissues to the CNS. Among these are nociceptive neurons that express calcitonin gene-related peptide-α (CGRPα). The role of peripheral CGRPα neurons (CANs) in acute and injury-induced pain has been studied using diphtheria toxin ablation, but their functional roles remain controversial. Because ablation permanently deletes a neuronal population, compensatory changes may ensue that mask the physiological or pathophysiological roles of CANs, particularly for injuries that occur after ablation. Therefore, we sought to define the role of intact CANs in vivo under baseline and injury conditions by using noninvasive transient optogenetic inhibition. We assessed pain behavior longitudinally from acute to chronic time points. We generated adult male and female mice that selectively express the outward rectifying proton pump archaerhodopsin-3 (Arch) in CANs, and inhibited their peripheral cutaneous terminals in models of neuropathic (spared nerve injury) and inflammatory (skin-muscle incision) pain using transdermal light activation of Arch. After nerve injury, brief activation of Arch reversed the chronic mechanical, cold, and heat hypersensitivity, alleviated the spontaneous pain, and reversed the sensitized mechanical currents in primary afferent somata. In contrast, Arch inhibition of CANs did not alter incision-induced hypersensitivity. Instead, incision-induced mechanical and heat hypersensitivity was alleviated by peripheral blockade of CGRPα peptide-receptor signaling. These results reveal that CANs have distinct roles in the time course of pain during neuropathic and incisional injuries and suggest that targeting peripheral CANs or CGRPα peptide-receptor signaling could selectively treat neuropathic or postoperative pain, respectively.SIGNIFICANCE STATEMENT The contribution of sensory afferent CGRPα neurons (CANs) to neuropathic and inflammatory pain is controversial. Here, we left CANs intact during neuropathic and perioperative incision injury by using transient transdermal optogenetic inhibition of CANs. We found that peripheral CANs are required for neuropathic mechanical, cold, and heat hypersensitivity, spontaneous pain, and sensitization of mechanical currents in afferent somata. However, they are dispensable for incisional pain transmission. In contrast, peripheral pharmacological inhibition of CGRPα peptide-receptor signaling alleviated the incisional mechanical and heat hypersensitivity, but had no effect on neuropathic pain. These results show that CANs have distinct roles in neuropathic and incisional pain and suggest that their targeting via novel peripheral treatments may selectively alleviate neuropathic versus incisional pain.
Keywords: archaerhodopsin; incision; inflammation; mechanotransduction; nerve injury; nociceptor.
Copyright © 2018 the authors 0270-6474/18/385807-19$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
