Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43
- PMID: 29925652
- PMCID: PMC6096804
- DOI: 10.1128/JVI.00404-18
Axonal Transport Enables Neuron-to-Neuron Propagation of Human Coronavirus OC43
Abstract
Human coronaviruses (HCoVs) are recognized respiratory pathogens for which accumulating evidence indicates that in vulnerable patients the infection can cause more severe pathologies. HCoVs are not always confined to the upper respiratory tract and can invade the central nervous system (CNS) under still unclear circumstances. HCoV-induced neuropathologies in humans are difficult to diagnose early enough to allow therapeutic interventions. Making use of our already described animal model of HCoV neuropathogenesis, we describe the route of neuropropagation from the nasal cavity to the olfactory bulb and piriform cortex and then the brain stem. We identified neuron-to-neuron propagation as one underlying mode of virus spreading in cell culture. Our data demonstrate that both passive diffusion of released viral particles and axonal transport are valid propagation strategies used by the virus. We describe for the first time the presence along axons of viral platforms whose static dynamism is reminiscent of viral assembly sites. We further reveal that HCoV OC43 modes of propagation can be modulated by selected HCoV OC43 proteins and axonal transport. Our work, therefore, identifies processes that may govern the severity and nature of HCoV OC43 neuropathogenesis and will make possible the development of therapeutic strategies to prevent occurrences.IMPORTANCE Coronaviruses may invade the CNS, disseminate, and participate in the induction of neurological diseases. Their neuropathogenicity is being increasingly recognized in humans, and the presence and persistence of human coronaviruses (HCoV) in human brains have been proposed to cause long-term sequelae. Using our mouse model relying on natural susceptibility to HCoV OC43 and neuronal cell cultures, we have defined the most relevant path taken by HCoV OC43 to access and spread to and within the CNS toward the brain stem and spinal cord and studied in cell culture the underlying modes of intercellular propagation to better understand its neuropathogenesis. Our data suggest that axonal transport governs HCoV OC43 egress in the CNS, leading to the exacerbation of neuropathogenesis. Exploiting knowledge on neuroinvasion and dissemination will enhance our ability to control viral infection within the CNS, as it will shed light on underlying mechanisms of neuropathogenesis and uncover potential druggable molecular virus-host interfaces.
Keywords: central nervous system; coronavirus; encephalitis; neuroinvasion; neuropathogenesis; neuropropagation.
Copyright © 2018 American Society for Microbiology.
Figures
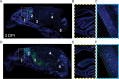



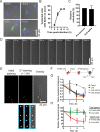
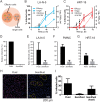

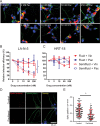
References
-
- Talbot P, Jacomy H, Desforges M. 2008. Pathogenesis of human coronaviruses other than severe acute respiratory syndrome coronavirus, p 313–324. In Perlman S, Gallagher T, Snijder EJ (ed.), Nidoviruses. ASM Press, Washington, DC.
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, Berger A, Burguiere AM, Cinatl J, Eickmann M, Escriou N, Grywna K, Kramme S, Manuguerra JC, Muller S, Rickerts V, Sturmer M, Vieth S, Klenk HD, Osterhaus AD, Schmitz H, Doerr HW. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 348:1967–1976. doi: 10.1056/NEJMoa030747. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

