Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype
- PMID: 29925654
- PMCID: PMC6096819
- DOI: 10.1128/JVI.01006-18
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype
Abstract
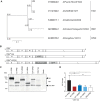
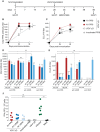
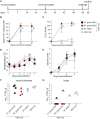
Immune responses induced by currently licensed inactivated influenza vaccines are mainly directed against the hemagglutinin (HA) glycoprotein, the immunodominant antigen of influenza viruses. The resulting antigenic drift of HA requires frequent updating of the vaccine composition and annual revaccination. On the other hand, the levels of antibodies directed against the neuraminidase (NA) glycoprotein, the second major influenza virus antigen, vary greatly. To investigate the potential of the more conserved NA protein for the induction of subtype-specific protection, vesicular stomatitis virus-based replicons expressing a panel of N1 proteins from prototypic seasonal and pandemic H1N1 strains and human H5N1 and H7N9 isolates were generated. Immunization of mice and ferrets with the replicon carrying the matched N1 protein resulted in robust humoral and cellular immune responses and protected against challenge with the homologous influenza virus with an efficacy similar to that of the matched HA protein, illustrating the potential of the NA protein as a vaccine antigen. The extent of protection after immunization with mismatched N1 proteins correlated with the level of cross-reactive neuraminidase-inhibiting antibody titers. Passive serum transfer experiments in mice confirmed that these functional antibodies determine subtype-specific cross-protection. Our findings illustrate the potential of NA-specific immunity for achieving broader protection against antigenic drift variants or newly emerging viruses carrying the same NA but a different HA subtype.IMPORTANCE Despite the availability of vaccines, annual influenza virus epidemics cause 250,000 to 500,000 deaths worldwide. Currently licensed inactivated vaccines, which are standardized for the amount of the hemagglutinin (HA) antigen, primarily induce strain-specific antibodies, whereas the immune response to the neuraminidase (NA) antigen, which is also present on the viral surface, is usually low. Using NA-expressing single-cycle vesicular stomatitis virus replicons, we show that the NA antigen conferred protection of mice and ferrets against not only the matched influenza virus strains but also viruses carrying NA proteins from other strains of the same subtype. The extent of protection correlated with the level of cross-reactive NA-inhibiting antibodies. This highlights the potential of the NA antigen for the development of more broadly protective influenza vaccines. Such vaccines may also provide partial protection against newly emerging strains with the same NA but a different HA subtype.
Keywords: VSV replicon vaccine platform; correlates of protection; functional antibodies; influenza A virus; neuraminidase protein.
Copyright © 2018 American Society for Microbiology.
Figures



Similar articles
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Cross-Reactive Neuraminidase-Inhibiting Antibodies Elicited by Immunization with Recombinant Neuraminidase Proteins of H5N1 and Pandemic H1N1 Influenza A Viruses.J Virol. 2015 Jul;89(14):7224-34. doi: 10.1128/JVI.00585-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948745 Free PMC article.
-
Inactivated H7 Influenza Virus Vaccines Protect Mice despite Inducing Only Low Levels of Neutralizing Antibodies.J Virol. 2017 Sep 27;91(20):e01202-17. doi: 10.1128/JVI.01202-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768855 Free PMC article.
-
Progress in the Development of Universal Influenza Vaccines.Viruses. 2020 Sep 17;12(9):1033. doi: 10.3390/v12091033. Viruses. 2020. PMID: 32957468 Free PMC article. Review.
-
Contribution of antibody production against neuraminidase to the protection afforded by influenza vaccines.Rev Med Virol. 2012 Jul;22(4):267-79. doi: 10.1002/rmv.1713. Epub 2012 Mar 22. Rev Med Virol. 2012. PMID: 22438243 Free PMC article. Review.
Cited by
-
Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains.J Virol. 2021 Aug 10;95(17):e0075921. doi: 10.1128/JVI.00759-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34160258 Free PMC article.
-
Anti-neuraminidase immunity in the combat against influenza.Expert Rev Vaccines. 2024 Jan-Dec;23(1):474-484. doi: 10.1080/14760584.2024.2343689. Epub 2024 Apr 23. Expert Rev Vaccines. 2024. PMID: 38632930 Free PMC article. Review.
-
A protective measles virus-derived vaccine inducing long-lasting immune responses against influenza A virus H7N9.NPJ Vaccines. 2023 Mar 24;8(1):46. doi: 10.1038/s41541-023-00643-9. NPJ Vaccines. 2023. PMID: 36964176 Free PMC article.
-
A neuraminidase-based inactivated influenza virus vaccine significantly reduced virus replication and pathology following homologous challenge in swine.Vaccine. 2025 Feb 6;46:126574. doi: 10.1016/j.vaccine.2024.126574. Epub 2024 Dec 7. Vaccine. 2025. PMID: 39645432
-
Engineered influenza virions reveal the contributions of non-hemagglutinin structural proteins to vaccine mediated protection.J Virol. 2021 Apr 26;95(10):e02021-20. doi: 10.1128/JVI.02021-20. Epub 2021 Mar 3. J Virol. 2021. PMID: 33658342 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

