Enhancing IgG distribution to lung mucosal tissue improves protective effect of anti-Pseudomonas aeruginosa antibodies
- PMID: 29925682
- PMCID: PMC6124411
- DOI: 10.1172/jci.insight.97844
Enhancing IgG distribution to lung mucosal tissue improves protective effect of anti-Pseudomonas aeruginosa antibodies
Abstract
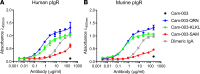
IgG antibodies are abundantly present in the vasculature but to a much lesser extent in mucosal tissues. This contrasts with antibodies of the IgA and IgM isotype that are present at high concentration in mucosal secretions due to active delivery by the polymeric Ig receptor (pIgR). IgG is the preferred isotype for therapeutic mAb development due to its long serum half-life and robust Fc-mediated effector function, and it is utilized to treat a diverse array of diseases with antigen targets located in the vasculature, serosa, and mucosa. As therapeutic IgG antibodies targeting the luminal side of mucosal tissue lack an active transport delivery mechanism, we sought to generate IgG antibodies that could be transported via pIgR, similarly to dimeric IgA and pentameric IgM. We show that an anti-Pseudomonas aeruginosa IgG fused with pIgR-binding peptides gained the ability to transcytose and be secreted via pIgR. Consistent with these results, pIgR-binding IgG antibodies exhibit enhanced localization to the bronchoalveolar space when compared with the parental IgG antibody. Furthermore, pIgR-binding mAbs maintained Fc-mediated functional activity and promoted enhanced survival compared with the parental mAb in a P. aeruginosa acute pneumonia model. Our results suggest that increasing IgG accumulation at mucosal surfaces by pIgR-mediated active transport can improve the efficacy of therapeutic mAbs that act at these sites.
Keywords: Bacterial infections; Immunoglobulins; Infectious disease; Pharmacology; Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

