Expression, Purification, and Characterization of a Novel Hybrid Peptide with Potent Antibacterial Activity
- PMID: 29925795
- PMCID: PMC6099547
- DOI: 10.3390/molecules23061491
Expression, Purification, and Characterization of a Novel Hybrid Peptide with Potent Antibacterial Activity
Abstract
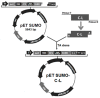
The hybrid peptide cecropin A (1⁻8)⁻LL37 (17⁻30) (C⁻L), derived from the sequence of cecropin A (C) and LL-37 (L), showed significantly increased antibacterial activity and minimized hemolytic activity than C and L alone. To obtain high-level production of C⁻L, the deoxyribonucleic acid sequence encoding C⁻L with preferred codons was cloned into pET-SUMO to construct a fusion expression vector, and overexpressed in Escherichia coli (E. coli) BL21 (DE3). The maximum fusion protein (92% purity) was obtained with the yield of 89.14 mg/L fermentation culture after purification with Ni-NTA Sepharose column. The hybrid C⁻L was cleaved from the fusion protein by SUMO-protease, and 17.54 mg/L pure active C⁻L was obtained. Furthermore, the purified C⁻L showed identical antibacterial and hemolytic activity to synthesized C⁻L. Stability analysis results exhibited that the activity of C⁻L changed little below 80 °C for 20 min, but when the temperature exceeded 80 °C, a significant decrease was observed. Varying the pH from 5.0 to 10.0 did not appear to influence the activity of C⁻L, however, pH below 4.0 decreased the antibacterial activity of C⁻L rapidly. Under the challenge of several proteases (pepsin, trypsin, and proteinase K), the functional activity of C⁻L was maintained over 50%. In summary, this study not only supplied an effective approach for high-level production of hybrid peptide C⁻L, but paved the way for its further exploration in controlling infectious diseases of farm animals or even humans.
Keywords: Escherichia coli; antibacterial activity; fusion expression; hybrid peptide; stability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Santos N.A., Fernandes R.S., Sgardioli B.F., Ramos M.A.S., Piccoli J.P., Camargo I.L.B.C., Bauab T.M., Cilli E.M. Antibacterial activity of the non-cytotoxic peptide (p-BthTX-I)(2) and Its serum degradation product against multidrug-resistant bacteria. Molecules. 2017;22:1898. doi: 10.3390/molecules22111898. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

