SERPINB2 is a novel indicator of stem cell toxicity
- PMID: 29925837
- PMCID: PMC6010432
- DOI: 10.1038/s41419-018-0748-x
SERPINB2 is a novel indicator of stem cell toxicity
Abstract
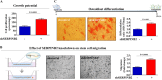
The toxicological evaluation of potential drug candidates is very important in the preclinical phase of drug development. Toxic materials may cause serious decline in stem cell function and loss of stemness. Indeed, we found that toxic exposure more profoundly suppressed the growth of stem cells than terminally differentiated fibroblasts. Importantly, toxic exposure suppressed stem cell migration and multi-lineage differentiation potential in vitro and in vivo. Moreover, early-response genes involved in stem cell properties such as self-renewal and differentiation capabilities can be used as specific markers to predict toxicity. In the present study, we also identified a labile toxic response gene, SERPINB2, which is significantly increased in response to various toxic agents in human stem cells in vitro and in vivo. Consistently, self-renewal, migration, and multi-lineage differentiation potential were markedly decreased following SERPINB2 overexpression. To the best of our knowledge, this is the first study to focus on the functions of SERPINB2 on the regenerative potential of stem cells in response to various existing chemicals, and the findings will facilitate the development of promising toxicity test platforms for newly developed chemicals.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

