A11, a novel diaryl acylhydrazone derivative, exerts neuroprotection against ischemic injury in vitro and in vivo
- PMID: 29925921
- PMCID: PMC6329839
- DOI: 10.1038/s41401-018-0028-4
A11, a novel diaryl acylhydrazone derivative, exerts neuroprotection against ischemic injury in vitro and in vivo
Abstract
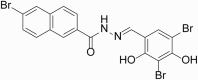
There is an urgent need to develop effective therapies for ischemic stroke, but the complicated pathological processes after ischemia make doing so difficult. In the current study, we identified a novel diaryl acylhydrazone derivative, A11, which has multiple neuroprotective properties in ischemic stroke models. First, A11 was demonstrated to induce neuroprotection against ischemic injury in a dose-dependent manner (from 0.3 to 3 μM) in three in vitro experimental ischemic stroke models: oxygen glucose deprivation (OGD), hydrogen peroxide, and glutamate-stimulated neuronal cell injury models. Moreover, A11 was able to potently alleviate three critical pathological changes, apoptosis, oxidative stress, and mitochondrial dysfunction, following ischemic insult in neuronal cells. Further analysis revealed that A11 upregulated the phosphorylation levels of protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) in OGD-exposed neuronal cells, suggesting joint activation of the phosphoinositide 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MEK)/ERK pathways. In rats with middle cerebral artery occlusion, single-dose administration of A11 (3 mg/kg per day, i.v.) at the onset of reperfusion significantly reduced the infarct volumes and ameliorated neurological deficits. Our study, for the first time, reports the anti-ischemic effect of diaryl acylhydrazone chemical entities, especially A11, which acts on multiple ischemia-associated pathological processes. Our results may provide new clues for the development of an effective therapeutic agent for ischemic stroke.
Keywords: diaryl acylhydrazone; extracellular signal-regulated kinase; ischemic stroke; middle cerebral artery occlusion; neuroprotection; oxygen glucose deprivation; protein kinase B.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
PDPOB Exerts Multiaspect Anti-Ischemic Effects Associated with the Regulation of PI3K/AKT and MAPK Signaling Pathways.ACS Chem Neurosci. 2021 Dec 1;12(23):4416-4427. doi: 10.1021/acschemneuro.1c00459. Epub 2021 Nov 10. ACS Chem Neurosci. 2021. PMID: 34755509
-
Isoquercetin attenuates oxidative stress and neuronal apoptosis after ischemia/reperfusion injury via Nrf2-mediated inhibition of the NOX4/ROS/NF-κB pathway.Chem Biol Interact. 2018 Mar 25;284:32-40. doi: 10.1016/j.cbi.2018.02.017. Epub 2018 Feb 16. Chem Biol Interact. 2018. PMID: 29454613
-
Quinolinyl Nitrone RP19 Induces Neuroprotection after Transient Brain Ischemia.ACS Chem Neurosci. 2017 Oct 18;8(10):2202-2213. doi: 10.1021/acschemneuro.7b00126. Epub 2017 Aug 9. ACS Chem Neurosci. 2017. PMID: 28731692
-
Central nervous system agents for ischemic stroke: neuroprotection mechanisms.Cent Nerv Syst Agents Med Chem. 2011 Jun 1;11(2):81-97. doi: 10.2174/187152411796011321. Cent Nerv Syst Agents Med Chem. 2011. PMID: 21521165 Free PMC article. Review.
-
Argon neuroprotection in ischemic stroke and its underlying mechanism.Brain Res Bull. 2024 Jun 15;212:110964. doi: 10.1016/j.brainresbull.2024.110964. Epub 2024 Apr 25. Brain Res Bull. 2024. PMID: 38670471 Review.
Cited by
-
An optically active isochroman-2H-chromene conjugate potently suppresses neuronal oxidative injuries associated with the PI3K/Akt and MAPK signaling pathways.Acta Pharmacol Sin. 2021 Jan;42(1):36-44. doi: 10.1038/s41401-020-0391-9. Epub 2020 May 11. Acta Pharmacol Sin. 2021. PMID: 32393798 Free PMC article.
-
Promoting Role of Long Non-Coding RNA Small Nucleolar RNA Host Gene 15 (SNHG15) in Neuronal Injury Following Ischemic Stroke via the MicroRNA-18a/CXC Chemokine Ligand 13 (CXCL13)/ERK/MEK Axis.Med Sci Monit. 2020 Aug 30;26:e923610. doi: 10.12659/MSM.923610. Med Sci Monit. 2020. PMID: 32862188 Free PMC article.
-
Endothelial cell-derived RSPO3 activates Gαi1/3-Erk signaling and protects neurons from ischemia/reperfusion injury.Cell Death Dis. 2023 Oct 7;14(10):654. doi: 10.1038/s41419-023-06176-2. Cell Death Dis. 2023. PMID: 37805583 Free PMC article.
-
Acylhydrazones and Their Biological Activity: A Review.Molecules. 2022 Dec 9;27(24):8719. doi: 10.3390/molecules27248719. Molecules. 2022. PMID: 36557851 Free PMC article. Review.
-
The impact of aging and oxidative stress in metabolic and nervous system disorders: programmed cell death and molecular signal transduction crosstalk.Front Immunol. 2023 Nov 8;14:1273570. doi: 10.3389/fimmu.2023.1273570. eCollection 2023. Front Immunol. 2023. PMID: 38022638 Free PMC article. Review.
References
-
- Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–e81. doi: 10.1016/S2214-109X(13)70089-5. - DOI - PMC - PubMed
-
- Buendia I, Tenti G, Michalska P, Mendez-Lopez I, Luengo E, Satriani M, et al. ITH14001, a CGP37157-nimodipine hybrid designed to regulate calcium homeostasis and oxidative stress, exerts neuroprotection in cerebral ischemia. ACS Chem Neurosci. 2017;8:67–81. doi: 10.1021/acschemneuro.6b00181. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

