Thermal carbonization in nanoscale reactors: controlled formation of carbon nanodots inside porous CaCO3 microparticles
- PMID: 29925932
- PMCID: PMC6010419
- DOI: 10.1038/s41598-018-27488-w
Thermal carbonization in nanoscale reactors: controlled formation of carbon nanodots inside porous CaCO3 microparticles
Abstract
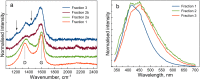
Synthesis of carbon nanodots (CNDs) in confined geometry via incorporation of dextran sulphate into pores of CaCO3 microparticles is demonstrated. The preparation process included three steps: co-precipitation of solutions of inorganic salts and carbon source, thermal treatment and CaCO3 matrix removal. We show that geometric constraints can be used to precisely control the amount of source material and to avoid formation of large carbon particles. Analysis of TEM data shows particle size of ~3.7 nm with narrow size distribution. Furthermore, we found that variation in pore morphology has a clear effect on CNDs structure and optical properties. CNDs with graphene oxide like structure were obtained in the nanoporous outer shell layer of CaCO3 microparticles, while less ordered CNDs with the evidence of complex disordered carbons were extracted from the inner microcavity. These results suggest that confined volume synthesis route in CaCO3 nanopores can be used to precisely control the structure and optical properties of CNDs.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hola K, et al. Carbon dots—Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today. 2014;9:590–603. doi: 10.1016/j.nantod.2014.09.004. - DOI
-
- Wang F, Xie Z, Zhang H, Liu C-Y, Zhang Y-G. Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv. Funct. Mater. 2011;21:1027–1031. doi: 10.1002/adfm.201002279. - DOI
-
- Li H, et al. One-step ultrasonic synthesis of water-soluble carbon nanoparticles with excellent photoluminescent properties. Carbon. 2011;49(2):605–609. doi: 10.1016/j.carbon.2010.10.004. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

