Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial
- PMID: 29925934
- PMCID: PMC6035166
- DOI: 10.1038/s41416-018-0132-8
Circulating biomarkers during treatment in patients with advanced biliary tract cancer receiving cediranib in the UK ABC-03 trial
Abstract
Background: Advanced biliary tract cancer (ABC) has a poor prognosis. Cediranib, in addition to cisplatin/gemcitabine [CisGem], improved the response rate, but did not improve the progression-free survival (PFS) in the ABC-03 study. Minimally invasive biomarkers predictive of cediranib benefit may improve patient outcomes.
Methods: Changes in 15 circulating plasma angiogenesis or inflammatory-related proteins and cytokeratin-18 (CK18), measured at baseline and during therapy until disease progression, were correlated with overall survival (OS) using time-varying covariate Cox models (TVC).
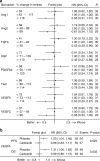
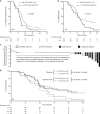
Results: Samples were available from n = 117/124 (94%) patients. Circulating Ang1&2, FGFb, PDGFbb, VEGFC, VEGFR1 and CK18 decreased as a result of the therapy, independent of treatment with cediranib. Circulating VEGFR2 and Tie2 were preferentially reduced by cediranib. Patients with increasing levels of VEGFA at any time had a worse PFS and OS; this detrimental effect was attenuated in patients receiving cediranib. TVC analysis revealed CK18 and VEGFR2 increases correlated with poorer OS in all patients (P < 0.001 and P = 0.02, respectively).
Conclusions: Rising circulating VEGFA levels in patients with ABC, treated with CisGem, are associated with worse PFS and OS, not seen in patients receiving cediranib. Rising levels of markers of tumour burden (CK18) and potential resistance (VEGFR2) are associated with worse outcomes and warrant validation.
Conflict of interest statement
S.B. has grants and non-financial support from AstraZeneca, outside this work. J.A.B. received personal fees from AstraZeneca during the conduct of the study. D.C. received grants from AstraZeneca, Amgen, Celgene, Merck Serono, Sanofi, Merrimack. and Medimmune, outside this work. H.W. participates in advisory boards for Lilly and AstraZeneca outside this work. J.S.W. has non-financial support from Novartis and Ipsen Pharma and personal fees from Roche outside this work. J.W.V. reports personal fees from Pfizer, grants, personal fees, and non-financial support from Novartis, personal fees from Abbott, personal fees and non-financial support from Celgene, personal fees from SIRTex, personal fees and non-financial support from Ipsen, grants, and personal fees from AstraZeneca Pharmaceuticals, outside the submitted work. D.A.A., A.C.B., P.C., C.D., M.D., A.L., S.M., A.M., M.M.N., K.M., D.H.P., C.R., P.J.R. and W.P.S. declare no competing interests.
Figures


References
-
- Bragazzi MC, et al. Cholangiocarcinoma: epidemiology and risk factors. Transl. Gastrointest. Cancer. 2012;1:21–32.
-
- Primrose JN, et al. Adjuvant capecitabine for biliary tract cancer: The BILCAP randomized study. J. Clin. Oncol. 2017;35(15_suppl):4006–4006. doi: 10.1200/JCO.2017.35.15_suppl.4006. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

