Decarboxylative sp3 C-N coupling via dual copper and photoredox catalysis
- PMID: 29925943
- PMCID: PMC6106865
- DOI: 10.1038/s41586-018-0234-8
Decarboxylative sp3 C-N coupling via dual copper and photoredox catalysis
Abstract
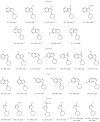
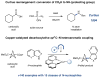
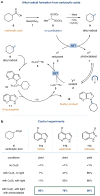
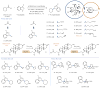
Over the past three decades, considerable progress has been made in the development of methods to construct sp2 carbon-nitrogen (C-N) bonds using palladium, copper or nickel catalysis1,2. However, the incorporation of alkyl substrates to form sp3 C-N bonds remains one of the major challenges in the field of cross-coupling chemistry. Here we demonstrate that the synergistic combination of copper catalysis and photoredox catalysis can provide a general platform from which to address this challenge. This cross-coupling system uses naturally abundant alkyl carboxylic acids and commercially available nitrogen nucleophiles as coupling partners. It is applicable to a wide variety of primary, secondary and tertiary alkyl carboxylic acids (through iodonium activation), as well as a vast array of nitrogen nucleophiles: nitrogen heterocycles, amides, sulfonamides and anilines can undergo C-N coupling to provide N-alkyl products in good to excellent efficiency, at room temperature and on short timescales (five minutes to one hour). We demonstrate that this C-N coupling protocol proceeds with high regioselectivity using substrates that contain several amine groups, and can also be applied to complex drug molecules, enabling the rapid construction of molecular complexity and the late-stage functionalization of bioactive pharmaceuticals.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Bhunia S, Pawar GG, Kumar SV, Jiang Y, Ma D. Selected Copper-Based Reaction for C–N, C–O, C–S, and C–C Bond Formation. Angew Chem Int Ed. 2017;56:16136–16179. - PubMed
-
- Knölker H-J, editor. The Alkaloids: Chemistry and Biology; Vol. 70. Elsevier; San Diego: 2011. - PubMed
-
- Vitaku E, Smith DT, Njardarson JT. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J Med Chem. 2014;57:10257–10274. - PubMed
-
- Ćirić-Marjanović G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth Met. 2013;177:1–47.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

