Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis
- PMID: 29926099
- PMCID: PMC6481553
- DOI: 10.1001/jamacardio.2018.1470
Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis
Abstract
Importance: Human genetic studies have indicated that plasma lipoprotein(a) (Lp[a]) is causally associated with the risk of coronary heart disease (CHD), but randomized trials of several therapies that reduce Lp(a) levels by 25% to 35% have not provided any evidence that lowering Lp(a) level reduces CHD risk.
Objective: To estimate the magnitude of the change in plasma Lp(a) levels needed to have the same evidence of an association with CHD risk as a 38.67-mg/dL (ie, 1-mmol/L) change in low-density lipoprotein cholesterol (LDL-C) level, a change that has been shown to produce a clinically meaningful reduction in the risk of CHD.
Design, setting, and participants: A mendelian randomization analysis was conducted using individual participant data from 5 studies and with external validation using summarized data from 48 studies. Population-based prospective cohort and case-control studies featured 20 793 individuals with CHD and 27 540 controls with individual participant data, whereas summarized data included 62 240 patients with CHD and 127 299 controls. Data were analyzed from November 2016 to March 2018.
Exposures: Genetic LPA score and plasma Lp(a) mass concentration.
Main outcomes and measures: Coronary heart disease.
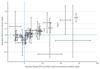
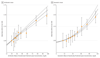
Results: Of the included study participants, 53% were men, all were of white European ancestry, and the mean age was 57.5 years. The association of genetically predicted Lp(a) with CHD risk was linearly proportional to the absolute change in Lp(a) concentration. A 10-mg/dL lower genetically predicted Lp(a) concentration was associated with a 5.8% lower CHD risk (odds ratio [OR], 0.942; 95% CI, 0.933-0.951; P = 3 × 10-37), whereas a 10-mg/dL lower genetically predicted LDL-C level estimated using an LDL-C genetic score was associated with a 14.5% lower CHD risk (OR, 0.855; 95% CI, 0.818-0.893; P = 2 × 10-12). Thus, a 101.5-mg/dL change (95% CI, 71.0-137.0) in Lp(a) concentration had the same association with CHD risk as a 38.67-mg/dL change in LDL-C level. The association of genetically predicted Lp(a) concentration with CHD risk appeared to be independent of changes in LDL-C level owing to genetic variants that mimic the relationship of statins, PCSK9 inhibitors, and ezetimibe with CHD risk.
Conclusions and relevance: The clinical benefit of lowering Lp(a) is likely to be proportional to the absolute reduction in Lp(a) concentration. Large absolute reductions in Lp(a) of approximately 100 mg/dL may be required to produce a clinically meaningful reduction in the risk of CHD similar in magnitude to what can be achieved by lowering LDL-C level by 38.67 mg/dL (ie, 1 mmol/L).
Conflict of interest statement
Figures

Comment in
-
Mendelian Randomization Evidence for Cardiovascular Precision Medicine.JAMA Cardiol. 2018 Jul 1;3(7):627-628. doi: 10.1001/jamacardio.2018.1543. JAMA Cardiol. 2018. PMID: 29926078 No abstract available.
References
-
- Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for coronary artery disease. Am J Cardiol. 1998;82(12A):57U–66U. - PubMed
-
- Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. - PubMed
-
- Clarke R, Peden JF, Hopewell JC, et al. PROCARDIS Consortium Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 204623/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- RG/08/014/BHF_/British Heart Foundation/United Kingdom
- RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- DH_/Department of Health/United Kingdom
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00002/7/MRC_/Medical Research Council/United Kingdom
- MR/S003746/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- SP/09/002/BHF_/British Heart Foundation/United Kingdom
- G0800270/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

