Andexanet Alfa: First Global Approval
- PMID: 29926311
- PMCID: PMC6061403
- DOI: 10.1007/s40265-018-0940-4
Andexanet Alfa: First Global Approval
Erratum in
-
Correction to: Andexanet Alfa: First Global Approval.Drugs. 2018 Aug;78(12):1285. doi: 10.1007/s40265-018-0954-y. Drugs. 2018. PMID: 30032379 Free PMC article.
Abstract
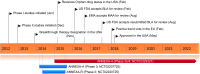
Intravenous andexanet alfa [coagulation factor Xa (recombinant), inactivated-zhzo; Andexxa®] is a first-in-class recombinant modified factor Xa protein that has been developed by Portola Pharmaceuticals as a universal antidote to reverse anticoagulant effects of direct or indirect factor Xa inhibitors. In May 2018, andexanet alfa received its first global approval in the USA for use in patients treated with rivaroxaban and apixaban, when reversal of anticoagulant effects is required in life-threatening or uncontrolled bleeding. Intravenous andexanet alfa is under regulatory review in the EU and is undergoing clinical development in Japan. This article summarizes the milestones in the development of andexanet alfa leading to this first global approval for reversing anticoagulation of rivaroxaban and apixaban in adults.
Conflict of interest statement
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. Young-A Heo is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Figures
References
-
- US FDA. FDA approved betrixaban (Bevyxxa, Portola) for the prophylaxis of venous thromboembolism (VTE) in adult patients [media release]. 23 June 2017. http://www.fda.gov.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical