A pharmacokinetics phase 1 bioequivalence study of the trastuzumab biosimilar MYL-1401O vs. EU-trastuzumab and US-trastuzumab
- PMID: 29926514
- PMCID: PMC6138509
- DOI: 10.1111/bcp.13689
A pharmacokinetics phase 1 bioequivalence study of the trastuzumab biosimilar MYL-1401O vs. EU-trastuzumab and US-trastuzumab
Abstract
Aims: Trastuzumab is a humanized monoclonal antibody that binds the human epidermal growth factor receptor 2 (HER2) oncoprotein and is an effective therapy for HER2-overexpressing breast cancer. MYL-1401O is a trastuzumab biosimilar. Here, we report results from a phase 1 study that investigated bioequivalence among MYL-1401O, reference EU-trastuzumab and US-trastuzumab.
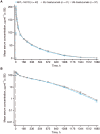
Methods: This single-centre, randomized, double-blind, three-arm, parallel-group, phase 1 study was conducted in healthy adult male volunteers. Subjects were randomized 1:1:1 to receive a single 8 mg kg-1 dose of MYL-1401O, EU-trastuzumab or US-trastuzumab as a 90-min intravenous infusion. The primary objective was to assess PK similarity among all three products. Primary endpoints assessed were peak serum concentration (Cmax), area under the serum concentration-time curve from time of dosing to time of last quantifiable concentration and from time of dosing to infinity. Secondary endpoints included time of Cmax, elimination rate constant, half-life, safety and immunogenicity.
Results: Of 132 subjects enrolled (44/treatment), 120 (MYL-1401O, n = 42; EU-trastuzumab, n = 41; US-trastuzumab, n = 37) were included in the PK analysis. The 90% confidence intervals of the ratios of geometric means for the primary endpoints were bounded within the predefined bioequivalence criterion of 80-125%. Secondary endpoints time of Cmax, elimination rate constant and half-life were similar among groups. All treatment-emergent adverse events were mild or moderate, similar across groups and no serious adverse events were reported. No treatment-related antidrug antibodies were detected.
Conclusions: MYL-1401O was well tolerated and demonstrated PK and safety profiles similar to EU-trastuzumab and US-trastuzumab in healthy volunteers (ClinicalTrials.gov, NCT02594761).
Keywords: bioequivalence; breast cancer; cancer; pharmacokinetics; phase 1.
© 2018 The British Pharmacological Society.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

