Incidence of Adverse Effects and Discontinuation Rate between Patients Receiving 250 Micrograms and 500 Micrograms of Roflumilast: A Comparative Study
- PMID: 29926552
- PMCID: PMC6148098
- DOI: 10.4046/trd.2018.0015
Incidence of Adverse Effects and Discontinuation Rate between Patients Receiving 250 Micrograms and 500 Micrograms of Roflumilast: A Comparative Study
Abstract
Background: Roflumilast is the only approved oral phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease (COPD) in patients with chronic bronchitis and a history of frequent exacerbations. The purpose of this study was to examine the incidence of adverse effects associated with roflumilast treatment in a real-world setting. Further, we compared the incidence of adverse effects and the discontinuation rate among patients receiving different doses.
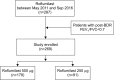
Methods: We identified all outpatients diagnosed with COPD at Seoul St. Mary's Hospital between May 2011 and September 2016 and retrospectively reviewed their medical records. Roflumilast was prescribed to patients in doses of 500 μg and 250 μg.
Results: A total of 269 COPD patients were prescribed roflumilast in our hospital during the study period. Among them, 178 patients were treated with 500 μg and 91 patients were treated with 250 μg. The incidence of adverse effects was 38.2% in the 500 μg group and 25.3% in the 250 μg group (p=0.034). The discontinuation rate of roflumilast was 41.6% (n=74) in the 500 μg group and 23.1% (n=21) in the 250 μg group (p=0.003). When adjusted by age, sex, smoking status, and lung function, 500 μg dose was significantly associated with the discontinuation of roflumilast (odds ratio, 2.87; p<0.001).
Conclusion: There was a lower incidence of adverse effects and discontinuation among patients treated with 250 μg compared with 500 μg dose. Further studies regarding the optimal dose of roflumilast are required.
Keywords: Adverse Effects; Pulmonary Disease, Chronic Obstructive; Roflumilast; Safety.
Copyright©2018. The Korean Academy of Tuberculosis and Respiratory Diseases.
Conflict of interest statement
Rhee CK received consulting/lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok.
Figures


References
-
- Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast: a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23:235–256. - PubMed
-
- Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast: an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005;366:563–571. - PubMed
-
- Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:154–161. - PubMed
-
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. - PubMed
-
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374:695–703. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

