Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions
- PMID: 29926770
- PMCID: PMC6238607
- DOI: 10.1089/ten.TEA.2018.0011
Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions
Abstract
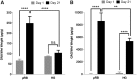
Hydrogels (HGs) are attractive matrices for cell-based cartilage tissue regeneration given their injectability and ability to fill defects with irregular shapes. However, most HGs developed to date often lack cell scale macroporosity, which restrains the encapsulated cells, leading to delayed new extracellular matrix deposition restricted to pericellular regions. Furthermore, tissue-engineered cartilage using conventional HGs generally suffers from poor mechanical property and fails to restore the load-bearing property of articular cartilage. The goal of this study was to evaluate the potential of macroporous gelatin-based microribbon (μRB) HGs as novel 3D matrices for accelerating chondrogenesis and new cartilage formation by human mesenchymal stem cells (MSCs) in 3D with improved mechanical properties. Unlike conventional HGs, these μRB HGs are inherently macroporous and exhibit cartilage-mimicking shock-absorbing mechanical property. After 21 days of culture, MSC-seeded μRB scaffolds exhibit a 20-fold increase in compressive modulus to 225 kPa, a range that is approaching the level of native cartilage. In contrast, HGs only resulted in a modest increase in compressive modulus of 65 kPa. Compared with conventional HGs, macroporous μRB scaffolds significantly increased the total amount of neocartilage produced by MSCs in 3D, with improved interconnectivity and mechanical strength. Altogether, these results validate gelatin-based μRBs as promising scaffolds for enhancing and accelerating MSC-based cartilage regeneration and may be used to enhance cartilage regeneration using other cell types as well.
Keywords: cartilage; gelatin; hydrogels; macroporous; mesenchymal stem cells; three-dimensional.
Conflict of interest statement
No competing financial interests exist.
Figures




Similar articles
-
Gelatin-Based Microribbon Hydrogels Support Robust MSC Osteogenesis across a Broad Range of Stiffness.ACS Biomater Sci Eng. 2020 Jun 8;6(6):3454-3463. doi: 10.1021/acsbiomaterials.9b01792. Epub 2020 May 27. ACS Biomater Sci Eng. 2020. PMID: 33463171 Free PMC article.
-
Spatially patterned microribbon-based hydrogels induce zonally-organized cartilage regeneration by stem cells in 3D.Acta Biomater. 2020 Jan 1;101:196-205. doi: 10.1016/j.actbio.2019.10.025. Epub 2019 Oct 19. Acta Biomater. 2020. PMID: 31634627
-
Mixed Composition Microribbon Hydrogels Induce Rapid and Synergistic Cartilage Regeneration by Mesenchymal Stem Cells in 3D via Paracrine Signaling Exchange.ACS Biomater Sci Eng. 2020 Jul 13;6(7):4166-4178. doi: 10.1021/acsbiomaterials.0c00131. Epub 2020 Jun 5. ACS Biomater Sci Eng. 2020. PMID: 33463346 Free PMC article.
-
Research Progress of the Types and Preparation Techniques of Scaffold Materials in Cartilage Tissue Engineering.Curr Stem Cell Res Ther. 2018;13(7):583-590. doi: 10.2174/1574888X12666170718152611. Curr Stem Cell Res Ther. 2018. PMID: 28721819 Review.
-
Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering.J Orthop Res. 2018 Jan;36(1):52-63. doi: 10.1002/jor.23670. Epub 2017 Aug 11. J Orthop Res. 2018. PMID: 28763118 Review.
Cited by
-
Nanoparticle-Mediated TGF-β Release from Microribbon-Based Hydrogels Accelerates Stem Cell-Based Cartilage Formation In Vivo.Ann Biomed Eng. 2020 Jul;48(7):1971-1981. doi: 10.1007/s10439-020-02522-z. Epub 2020 May 6. Ann Biomed Eng. 2020. PMID: 32377980 Free PMC article.
-
The Effects of ROCK Inhibition on Mesenchymal Stem Cell Chondrogenesis Are Culture Model Dependent.Tissue Eng Part A. 2020 Feb;26(3-4):130-139. doi: 10.1089/ten.TEA.2019.0068. Epub 2019 Sep 20. Tissue Eng Part A. 2020. PMID: 31411113 Free PMC article.
-
Gelatin-Based Microribbon Hydrogels Support Robust MSC Osteogenesis across a Broad Range of Stiffness.ACS Biomater Sci Eng. 2020 Jun 8;6(6):3454-3463. doi: 10.1021/acsbiomaterials.9b01792. Epub 2020 May 27. ACS Biomater Sci Eng. 2020. PMID: 33463171 Free PMC article.
-
Vascular smooth muscle cells can be circumferentially aligned inside a channel using tunable gelatin microribbons.Biofabrication. 2024 Oct 30;17(1):10.1088/1758-5090/ad88a7. doi: 10.1088/1758-5090/ad88a7. Biofabrication. 2024. PMID: 39423834
-
Translational Applications of Hydrogels.Chem Rev. 2021 Sep 22;121(18):11385-11457. doi: 10.1021/acs.chemrev.0c01177. Epub 2021 May 3. Chem Rev. 2021. PMID: 33938724 Free PMC article. Review.
References
-
- Griffin T.M., and Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exer Sport Sci Rev 33, 195, 2005 - PubMed
-
- Wang T., Lai J.H., Han L.H., Tong X., and Yang F. Chondrogenic differentiation of adipose-derived stromal cells in combinatorial hydrogels containing cartilage matrix proteins with decoupled mechanical stiffness. Tissue Eng A 20, 2131, 2014 - PubMed
-
- Guilak F., Awad H.A., Fermor B., Leddy H.A., and Gimble J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 41, 389, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

