Salmonella Typhimurium Infection of Human Monocyte-Derived Macrophages
- PMID: 29927091
- PMCID: PMC6105500
- DOI: 10.1002/cpmc.56
Salmonella Typhimurium Infection of Human Monocyte-Derived Macrophages
Abstract
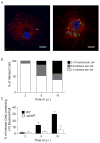
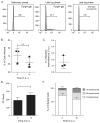
The successful infection of macrophages by non-typhoidal serovars of Salmonella enterica is likely essential to the establishment of the systemic disease they sometimes cause in susceptible human populations. However, the interactions between Salmonella and human macrophages are not widely studied, with mouse macrophages being a much more common model system. Fundamental differences between mouse and human macrophages make this less than ideal. Additionally, the inability of human macrophage-like cell lines to replicate some properties of primary macrophages makes the use of primary cells desirable. Here we present protocols to study the infection of human monocyte-derived macrophages with Salmonella Typhimurium. These include a method for differentiating monocyte-derived macrophages in vitro and protocols for infecting them with Salmonella Typhimurium, as well as assays to measure the extent of infection, replication, and death. These protocols are useful for the investigation of both bacterial and host factors that determine the outcome of infection. © 2018 by John Wiley & Sons, Inc.
Keywords: bacteria; infection; intracellular pathogen; macrophages; monocyte-derived macrophage; salmonella.
© 2018 John Wiley & Sons, Inc.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

