Chemical and Biochemical Perspectives of Protein Lysine Methylation
- PMID: 29927582
- PMCID: PMC6668730
- DOI: 10.1021/acs.chemrev.8b00008
Chemical and Biochemical Perspectives of Protein Lysine Methylation
Abstract
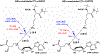
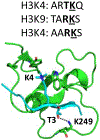
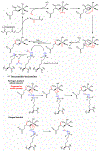
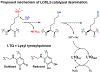
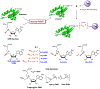
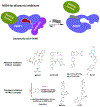
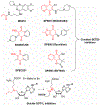
Protein lysine methylation is a distinct posttranslational modification that causes minimal changes in the size and electrostatic status of lysine residues. Lysine methylation plays essential roles in regulating fates and functions of target proteins in an epigenetic manner. As a result, substrates and degrees (free versus mono/di/tri) of protein lysine methylation are orchestrated within cells by balanced activities of protein lysine methyltransferases (PKMTs) and demethylases (KDMs). Their dysregulation is often associated with neurological disorders, developmental abnormalities, or cancer. Methyllysine-containing proteins can be recognized by downstream effector proteins, which contain methyllysine reader domains, to relay their biological functions. While numerous efforts have been made to annotate biological roles of protein lysine methylation, limited work has been done to uncover mechanisms associated with this modification at a molecular or atomic level. Given distinct biophysical and biochemical properties of methyllysine, this review will focus on chemical and biochemical aspects in addition, recognition, and removal of this posttranslational mark. Chemical and biophysical methods to profile PKMT substrates will be discussed along with classification of PKMT inhibitors for accurate perturbation of methyltransferase activities. Semisynthesis of methyllysine-containing proteins will also be covered given the critical need for these reagents to unambiguously define functional roles of protein lysine methylation.
Conflict of interest statement
The author declares no competing financial interest.
Figures



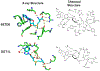
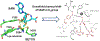

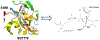
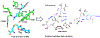
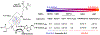








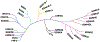









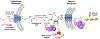


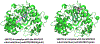
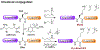


References
-
- Allis CD, and Jenuwein T The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet 2016, 17, 487–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

