Primary Deuterium Kinetic Isotope Effects: A Probe for the Origin of the Rate Acceleration for Hydride Transfer Catalyzed by Glycerol-3-Phosphate Dehydrogenase
- PMID: 29927590
- PMCID: PMC6091503
- DOI: 10.1021/acs.biochem.8b00536
Primary Deuterium Kinetic Isotope Effects: A Probe for the Origin of the Rate Acceleration for Hydride Transfer Catalyzed by Glycerol-3-Phosphate Dehydrogenase
Abstract
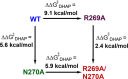
Large primary deuterium kinetic isotope effects (1° DKIEs) on enzyme-catalyzed hydride transfer may be observed when the transferred hydride tunnels through the energy barrier. The following 1° DKIEs on kcat/ Km and relative reaction driving force are reported for wild-type and mutant glycerol-3-phosphate dehydrogenase (GPDH)-catalyzed reactions of NADL (L = H, D): wild-type GPDH, ΔΔ G⧧ = 0 kcal/mol, 1° DKIE = 1.5; N270A, 5.6 kcal/mol, 3.1; R269A, 9.1 kcal/mol, 2.8; R269A + 1.0 M guanidine, 2.4 kcal/mol, 2.7; R269A/N270A, 11.5 kcal/mol, 2.4. Similar 1° DKIEs were observed on kcat. The narrow range of 1° DKIEs (2.4-3.1) observed for a 9.1 kcal/mol change in reaction driving force provides strong evidence that these are intrinsic 1° DKIEs on rate-determining hydride transfer. Evidence is presented that the intrinsic DKIE on wild-type GPDH-catalyzed reduction of DHAP lies in this range. A similar range of 1° DKIEs (2.4-2.9) on ( kcat/ KGA, M-1 s-1) was reported for dianion-activated hydride transfer from NADL to glycolaldehyde (GA) [Reyes, A. C.; Amyes, T. L.; Richard, J. P. J. Am. Chem. Soc. 2016, 138, 14526-14529]. These 1° DKIEs are much smaller than those observed for enzyme-catalyzed hydrogen transfer that occurs mainly by quantum mechanical tunneling. These results support the conclusion that the rate acceleration for GPDH-catalyzed reactions is due to the stabilization of the transition state for hydride transfer by interactions with the protein catalyst. The small 1° DKIEs reported for mutant GPDH-catalyzed and for wild-type dianion-activated reactions are inconsistent with a model where the dianion binding energy is utilized in the stabilization of a tunneling ready state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





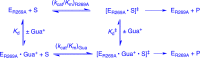


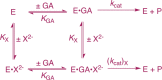


References
-
- Richard J. P.; Huber R. E.; Lin S.; Heo C.; Amyes T. L. (1996) Structure-reactivity relationships for β-galactosidase (Escherichia coli, lac Z). 3. Evidence that Glu-461 participates in Bronsted acid-base catalysis of β-D-galactopyranosyl group transfer. Biochemistry 35, 12377–12386. 10.1021/bi961028j. - DOI - PubMed
-
- Richard J. P.; Huber R. E.; Heo C.; Amyes T. L.; Lin S. (1996) Structure-reactivity relationships for β-galactosidase (Escherichia coli, lac Z). 4. Mechanism for reaction of nucleophiles with the galactosyl-enzyme intermediates of E461G and E461Q β-galactosidases. Biochemistry 35, 12387–12401. 10.1021/bi961029b. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

