Activation of TREK currents by riluzole in three subgroups of cultured mouse nodose ganglion neurons
- PMID: 29928032
- PMCID: PMC6013220
- DOI: 10.1371/journal.pone.0199282
Activation of TREK currents by riluzole in three subgroups of cultured mouse nodose ganglion neurons
Abstract
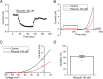
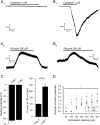
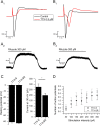
Two-pore domain potassium channels (K2P) constitute major candidates for the regulation of background potassium currents in mammalian cells. Channels of the TREK subfamily are also well positioned to play an important role in sensory transduction due to their sensitivity to a large number of physiological and physical stimuli (pH, mechanical, temperature). Following our previous report describing the molecular expression of different K2P channels in the vagal sensory system, here we confirm that TREK channels are functionally expressed in neurons from the mouse nodose ganglion (mNG). Neurons were subdivided into three groups (A, Ah and C) based on their response to tetrodotoxin and capsaicin. Application of the TREK subfamily activator riluzole to isolated mNG neurons evoked a concentration-dependent outward current in the majority of cells from all the three subtypes studied. Riluzole increased membrane conductance and hyperpolarized the membrane potential by approximately 10 mV when applied to resting neurons. The resting potential was similar in all three groups, but C cells were clearly less excitable and showed smaller hyperpolarization-activated currents at -100 mV and smaller sustained currents at -30 mV. Our results indicate that the TREK subfamily of K2P channels might play an important role in the maintenance of the resting membrane potential in sensory neurons of the autonomic nervous system, suggesting its participation in the modulation of vagal reflexes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Characterization of temperature-sensitive leak K+ currents and expression of TRAAK, TREK-1, and TREK2 channels in dorsal root ganglion neurons of rats.Mol Brain. 2018 Jul 6;11(1):40. doi: 10.1186/s13041-018-0384-5. Mol Brain. 2018. PMID: 29980241 Free PMC article.
-
PIP2 Mediated Inhibition of TREK Potassium Currents by Bradykinin in Mouse Sympathetic Neurons.Int J Mol Sci. 2020 Jan 8;21(2):389. doi: 10.3390/ijms21020389. Int J Mol Sci. 2020. PMID: 31936257 Free PMC article.
-
Activation of TREK currents by the neuroprotective agent riluzole in mouse sympathetic neurons.J Neurosci. 2011 Jan 26;31(4):1375-85. doi: 10.1523/JNEUROSCI.2791-10.2011. J Neurosci. 2011. PMID: 21273422 Free PMC article.
-
Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels.Int J Mol Sci. 2021 Aug 23;22(16):9062. doi: 10.3390/ijms22169062. Int J Mol Sci. 2021. PMID: 34445768 Free PMC article. Review.
-
Stretch-activated potassium currents in the heart: Focus on TREK-1 and arrhythmias.Prog Biophys Mol Biol. 2017 Nov;130(Pt B):223-232. doi: 10.1016/j.pbiomolbio.2017.05.005. Epub 2017 May 16. Prog Biophys Mol Biol. 2017. PMID: 28526352 Review.
Cited by
-
Pulmonary Sensory Receptors.Adv Anat Embryol Cell Biol. 2021;233:1-65. doi: 10.1007/978-3-030-65817-5_1. Adv Anat Embryol Cell Biol. 2021. PMID: 33950466 No abstract available.
-
Are TREK Channels Temperature Sensors?Front Cell Neurosci. 2021 Oct 6;15:744702. doi: 10.3389/fncel.2021.744702. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34690704 Free PMC article. Review.
-
Contribution of K2P Potassium Channels to Cardiac Physiology and Pathophysiology.Int J Mol Sci. 2021 Jun 21;22(12):6635. doi: 10.3390/ijms22126635. Int J Mol Sci. 2021. PMID: 34205717 Free PMC article. Review.
-
Serotonergic Modulation of Persistent Inward Currents in Serotonergic Neurons of Medulla in ePet-EYFP Mice.Front Neural Circuits. 2021 Apr 6;15:657445. doi: 10.3389/fncir.2021.657445. eCollection 2021. Front Neural Circuits. 2021. PMID: 33889077 Free PMC article.
-
Ion Channels and Thermosensitivity: TRP, TREK, or Both?Int J Mol Sci. 2019 May 14;20(10):2371. doi: 10.3390/ijms20102371. Int J Mol Sci. 2019. PMID: 31091651 Free PMC article. Review.
References
-
- Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore-Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist. 2003;9(1):46–56. doi: 10.1177/1073858402239590 . - DOI - PubMed
-
- Lamas JA. Mechanosensitive K2P channels, TREKking through the autonomic nervous system In: Kamkin A, Lozinsky I, editors. Mechanically gated channels and their regulation. Mechanosensitivity in cells and tissues. 6 Dordrecht: Springer Science+Business Media; 2012. p. 35–68.
-
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90(2):559–605. doi: 10.1152/physrev.00029.2009 . - DOI - PubMed
-
- Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol. 2010;298(2):G212–21. doi: 10.1152/ajpgi.00396.2009 . - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

