PPARγ is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis
- PMID: 29928066
- PMCID: PMC6013021
- DOI: 10.1371/journal.ppat.1007100
PPARγ is critical for Mycobacterium tuberculosis induction of Mcl-1 and limitation of human macrophage apoptosis
Abstract
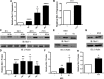
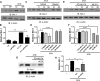
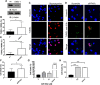
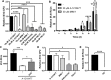
Peroxisome proliferator-activated receptor (PPAR)γ is a global transcriptional regulator associated with anti-inflammatory actions. It is highly expressed in alveolar macrophages (AMs), which are unable to clear the intracellular pathogen Mycobacterium tuberculosis (M.tb). Although M.tb infection induces PPARγ in human macrophages, which contributes to M.tb growth, the mechanisms underlying this are largely unknown. We undertook NanoString gene expression analysis to identify novel PPARγ effectors that condition macrophages to be more susceptible to M.tb infection. This revealed several genes that are differentially regulated in response to PPARγ silencing during M.tb infection, including the Bcl-2 family members Bax (pro-apoptotic) and Mcl-1 (pro-survival). Apoptosis is an important defense mechanism that prevents the growth of intracellular microbes, including M.tb, but is limited by virulent M.tb. This suggested that M.tb differentially regulates Mcl-1 and Bax expression through PPARγ to limit apoptosis. In support of this, gene and protein expression analysis revealed that Mcl-1 expression is driven by PPARγ during M.tb infection in human macrophages. Further, 15-lipoxygenase (15-LOX) is critical for PPARγ activity and Mcl-1 expression. We also determined that PPARγ and 15-LOX regulate macrophage apoptosis during M.tb infection, and that pre-clinical therapeutics that inhibit Mcl-1 activity significantly limit M.tb intracellular growth in both human macrophages and an in vitro TB granuloma model. In conclusion, identification of the novel PPARγ effector Mcl-1 has determined PPARγ and 15-LOX are critical regulators of apoptosis during M.tb infection and new potential targets for host-directed therapy for M.tb.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Mycobacterium tuberculosis: prePPARing and Maintaining the Replicative Niche.Trends Microbiol. 2018 Oct;26(10):813-814. doi: 10.1016/j.tim.2018.08.001. Epub 2018 Aug 14. Trends Microbiol. 2018. PMID: 30119946
References
-
- Glass CK, Saijo K. Nuclear receptor transrepressionpathways that regulate inflammationin macrophages and T cells. Nat Rev Immunol. Nature Publishing Group; 2010. May 1;10(5):365–76. doi: 10.1038/nri2748 - DOI - PubMed
-
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013. May;19(5):557–66. doi: 10.1038/nm.3159 - DOI - PMC - PubMed
-
- Rajaram MVS, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010. July 15;185(2):929–42. doi: 10.4049/jimmunol.1000866 - DOI - PMC - PubMed
-
- Mahajan S, Dkhar HK, Chandra V, Dave S, Nanduri R, Janmeja AK, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARγ and TR4 for survival. J Immunol. 2012. June 1;188(11):5593–603. doi: 10.4049/jimmunol.1103038 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

