Voltage-Gated Sodium Channels as Insecticide Targets
- PMID: 29928068
- PMCID: PMC6005695
- DOI: 10.1016/B978-0-12-417010-0.00005-7
Voltage-Gated Sodium Channels as Insecticide Targets
Abstract
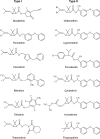
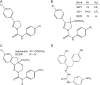
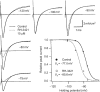
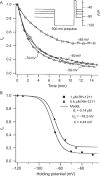
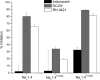
Voltage-gated sodium channels are critical for the generation and propagation of action potentials. They are the primary target of several classes of insecticides, including DDT, pyrethroids and sodium channel blocker insecticides (SCBIs). DDT and pyrethroids preferably bind to open sodium channels and stabilize the open state, causing prolonged currents. In contrast, SCBIs block sodium channels by binding to the inactivated state. Many sodium channel mutations are associated with knockdown resistance (kdr) to DDT and pyrethroids in diverse arthropod pests. Functional characterization of kdr mutations together with computational modelling predicts dual pyrethroid receptor sites on sodium channels. In contrast, the molecular determinants of the SCBI receptor site remain largely unknown. In this review, we summarize current knowledge about the molecular mechanisms of action of pyrethroids and SCBIs, and highlight the differences in the molecular interaction of these insecticides with insect versus mammalian sodium channels.
Figures




References
-
- Ahmad M, Hollingworth RM. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) Pest Manag. Sci. 2004;60:465–473. - PubMed
-
- Ahmad M, Hollingworth RM, Wise JC. Broad-spectrum insecticide resistance in obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) from Michigan. Pest Manag. Sci. 2002;58:834–838. - PubMed
-
- BASF Agricultural Products. Metaflumizone world-wide technical brochure 2007
-
- Bloomquist JR. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. - PubMed
-
- Bloomquist JR, Soderlund DM. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol. Pharmacol. 1988;33:543–550. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
