Pharmacokinetics and pharmacodynamics of linezolid in plasma/cerebrospinal fluid in patients with cerebral hemorrhage after lateral ventricular drainage by Monte Carlo simulation
- PMID: 29928111
- PMCID: PMC6001839
- DOI: 10.2147/DDDT.S168757
Pharmacokinetics and pharmacodynamics of linezolid in plasma/cerebrospinal fluid in patients with cerebral hemorrhage after lateral ventricular drainage by Monte Carlo simulation
Abstract
Objective: We investigated the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of linezolid in patients who had suffered cerebral hemorrhage after lateral ventricular drainage.
Materials and methods: Ten patients with cerebral hemorrhage after lateral ventricular drainage with stroke-associated pneumonia who were given linezolid were enrolled. Plasma and cerebrospinal fluid (CSF) samples were taken at appropriate intervals after the first administration of linezolid and assayed by high-performance liquid chromatography (HPLC). Then, PK parameters were estimated, and a Monte Carlo simulation was used to calculate the probability of target attainments (PTAs) for linezolid achieving the PK/PD index at different minimal inhibitory concentrations (MICs).
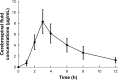
Results: The maximum concentration of linezolid in plasma and CSF was reached at 1.00 h and 3.10 h, respectively. The average penetration of linezolid in CSF was 56.81%. If the area under the plasma concentration vs time curve from zero to the final sampling time (AUC0-24 h)/MIC ≥ 59.1 was applied as a parameter, the PTA of linezolid in plasma could provide good coverage (PTA ≥ 90%) only for pathogens with a MIC of ≤2 μg/mL, whereas it could be achieved in CSF with a MIC of ≤1 μg/mL. If %T > MIC ≥ 40% was applied as a parameter, the PTA of linezolid in plasma/CSF could provide good coverage if the MIC was ≤4 μg/mL.
Conclusions: For patients with infection of the central nervous system and who are sensitive to the drug, the usual dosing regimens of linezolid can achieve a good therapeutic effect. However, for critically ill or drug-resistant patients, an increase in dose, the frequency of administration, or longer infusion may be needed to improve the curative effect.
Keywords: Monte Carlo simulation; cerebral hemorrhage; cerebrospinal fluid; linezolid; pharmacodynamics; pharmacokinetics; plasma.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Murthy SB, Moradiya Y, Shah J, Hanley DF, Ziai WC. Incidence, predictors, and outcomes of ventriculostomy-associated infections in spontaneous intracerebral hemorrhage. Neurocrit Care. 2016;24:389–396. - PubMed
-
- Yang ZJ, Zhong HL, Wang ZM, Zhao F, Liu PN. Prevention of postoperative intracranial infection in patients with cerebrospinal fluid rhinorrhea. Chin Med J (Engl) 2011;124:4189–4192. - PubMed
-
- Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, et al. Ventriculostomy-associated infections: incidence and risk factors. Am J Infect Cont. 2005;33:137–143. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

