Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of "Protein-Misfolding Cyclic Amplification" and "Real-Time Quaking-Induced Conversion" as Diagnostic Tools
- PMID: 29928254
- PMCID: PMC5997809
- DOI: 10.3389/fneur.2018.00415
Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of "Protein-Misfolding Cyclic Amplification" and "Real-Time Quaking-Induced Conversion" as Diagnostic Tools
Abstract
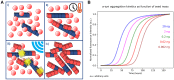
The accumulation and deposition of α-synuclein aggregates in brain tissue is the main event in the pathogenesis of different neurodegenerative disorders grouped under the term of synucleinopathies. They include Parkinson's disease, dementia with Lewy bodies and multiple system atrophy. To date, the diagnosis of any of these disorders mainly relies on the recognition of clinical symptoms, when the neurodegeneration is already in an advanced phase. In the last years, several efforts have been carried out to develop new diagnostic tools for early diagnosis of synucleinopathies, with special interest to Parkinson's disease. The Protein-Misfolding Cyclic Amplification (PMCA) and the Real-Time Quaking-Induced Conversion (RT-QuIC) are ultrasensitive protein amplification assays for the detection of misfolded protein aggregates. Starting from the successful application in the diagnosis of human prion diseases, these techniques were recently tested for the detection of misfolded α-synuclein in brain homogenates and cerebrospinal fluid samples of patients affected by synucleinopathies. So far, only a few studies on a limited number of samples have been performed to test PMCA and RT-QuIC diagnostic reliability. Neverthless, these assays have shown very high sensitivity and specificity in detecting synucleinopathies even at the pre-clinical stage. Despite the application of PMCA and RT-QuIC for α-synuclein detection in biological fluids is very recent, these techniques seem to have the potential for identifying subjects that will be likely to develop synucleinopathies.
Keywords: PMCA; RT-QuIC; early diagnosis; synucleinopathies; α-synuclein.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

