Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways
- PMID: 29928342
- PMCID: PMC6004715
- DOI: 10.3892/ol.2018.8584
Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways
Abstract
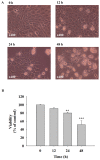
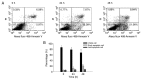
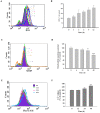
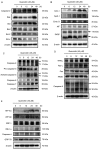
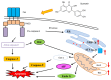
Oral cancer is a cause of cancer-associated mortality worldwide and the treatment of oral cancer includes radiation, surgery and chemotherapy. Quercetin is a component from natural plant products and it has been demonstrated that quercetin is able to induce cytotoxic effects through induction of cell apoptosis in a number of human cancer cell lines. However, there is no available information to demonstrate that quercetin is able to induce apoptosis in human oral cancer cells. In the present study, the effect of quercetin on the cell death via the induction of apoptosis in human oral cancer SAS cells was investigated using flow cytometry, Annexin V/propidium iodide (PI) double staining, western blotting and confocal laser microscopy examination, to test for cytotoxic effects at 6-48 h after treatment with quercetin. The rate of cell death increased with the duration of quercetin treatment based on the results of a cell viability assay, increased Annexin V/PI staining, increased reactive oxygen species and Ca2+ production, decreased the levels of mitochondrial membrane potential (ΔΨm), increased proportion of apoptotic cells and altered levels of apoptosis-associated protein expression in SAS cells. The results from western blotting revealed that quercetin increased Fas, Fas-Ligand, fas-associated protein with death domain and caspase-8, all of which associated with cell surface death receptor. Furthermore, quercetin increased the levels of activating transcription factor (ATF)-6α, ATF-6β and gastrin-releasing peptide-78 which indicated an increase in endoplasm reticulum stress, increased levels of the pro-apoptotic protein BH3 interacting-domain death antagonist, and decreased levels of anti-apoptotic proteins B-cell lymphoma (Bcl) 2 and Bcl-extra large which may have led to the decreases of ΔΨm. Additionally, confocal microscopy suggested that quercetin was able to increase the expression levels of cytochrome c, apoptosis-inducing factor and endonuclease G, which are associated with apoptotic pathways. Therefore, it is hypothesized that quercetin may potentially be used as a novel anti-cancer agent for the treatment of oral cancer in future.
Keywords: apoptosis; endoplasmic reticulum mediated signaling pathway; human oral cancer SAS cells; mitochondria signaling pathway; quercetin.
Figures


References
-
- Halboub ES, Al-Anazi YM, Al-Mohaya MA. Characterization of Yemeni patients treated for oral and pharyngeal cancers in Saudi Arabia. Saudi Med J. 2011;32:1177–1182. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
