Increased tumor vascularization is associated with the amount of immune competent PD-1 positive cells in testicular germ cell tumors
- PMID: 29928359
- PMCID: PMC6004709
- DOI: 10.3892/ol.2018.8597
Increased tumor vascularization is associated with the amount of immune competent PD-1 positive cells in testicular germ cell tumors
Abstract
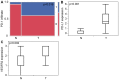
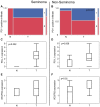
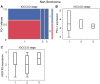
Testicular germ cell cancer in a metastatic state is curable with a cisplatin-based first line chemotherapy. However, 10-15% of these patients are resistant to first line chemotherapy and are thus left with only palliative options. Immunotherapies and inhibition of angiogenesis used in multiple types of cancer; however, the molecular context of angiogenesis and immune checkpoints in the development and progression of testicular cancers is still unknown. Therefore, the present study performed tissue micro array based analysis of 84 patients with immunohistochemistry of programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1) and vascular endothelial growth factor receptor 2 (VEGFR2) of testicular cancer and corresponding normal appearing testis tissue, matching the results with clinical data. The results demonstrated that PD-L1 was significantly upregulated in testicular tumors and that PD-1 positive cells significantly infiltrated the testicular tumor when compared with normal testicular tissue. VEGFR2 was significantly upregulated in testicular cancer. It was indicated that PD-1 expressing cytotoxic cells may require pathologic tumor vessels to pass the blood-testis-barrier in order to migrate into the tumor. Notably, when matching the clinical data for PD-1, PD-L1 and VEGFR2 there were no differences in expression in the different International Germ Cell Cancer Collaborative Group stages of non-seminoma. These data suggested that the anti-PD-1/PD-L1 immunotherapy and the anti-angiogenic therapy, sequentially or in combination, may be a promising option in the treatment of testicular cancer.
Keywords: immune; programmed cell death ligand 1; programmed cell death protein 1; testicular cancer; vascular endothelial growth factor receptor 2.
Figures



Similar articles
-
Frequent PD-L1 expression in testicular germ cell tumors.Br J Cancer. 2015 Jul 28;113(3):411-3. doi: 10.1038/bjc.2015.244. Epub 2015 Jul 14. Br J Cancer. 2015. PMID: 26171934 Free PMC article.
-
Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors.Ann Oncol. 2016 Feb;27(2):300-5. doi: 10.1093/annonc/mdv574. Epub 2015 Nov 23. Ann Oncol. 2016. PMID: 26598537 Free PMC article.
-
Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors.Oncotarget. 2017 Mar 28;8(13):21794-21805. doi: 10.18632/oncotarget.15585. Oncotarget. 2017. PMID: 28423520 Free PMC article.
-
Is There a Role for Programmed Death Ligand-1 Testing and Immunotherapy in Colorectal Cancer With Microsatellite Instability? Part II-The Challenge of Programmed Death Ligand-1 Testing and Its Role in Microsatellite Instability-High Colorectal Cancer.Arch Pathol Lab Med. 2018 Jan;142(1):26-34. doi: 10.5858/arpa.2017-0041-RA. Epub 2017 Nov 9. Arch Pathol Lab Med. 2018. PMID: 29120224 Review.
-
Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses.Acta Pharm Sin B. 2020 May;10(5):723-733. doi: 10.1016/j.apsb.2019.09.006. Epub 2019 Sep 25. Acta Pharm Sin B. 2020. PMID: 32528824 Free PMC article. Review.
Cited by
-
Endothelial cells in colorectal cancer.World J Gastrointest Oncol. 2019 Nov 15;11(11):946-956. doi: 10.4251/wjgo.v11.i11.946. World J Gastrointest Oncol. 2019. PMID: 31798776 Free PMC article. Review.
-
A Methylation-Based Reclassification of Bladder Cancer Based on Immune Cell Genes.Cancers (Basel). 2020 Oct 20;12(10):3054. doi: 10.3390/cancers12103054. Cancers (Basel). 2020. PMID: 33092083 Free PMC article.
-
Unraveling drug resistance mechanisms in testicular cancer through immune cell phenotypes: a bidirectional Mendelian randomization analysis revealing novel therapeutic targets.Discov Oncol. 2025 May 24;16(1):912. doi: 10.1007/s12672-025-02757-z. Discov Oncol. 2025. PMID: 40413316 Free PMC article.
-
Targeting the tumor stroma for cancer therapy.Mol Cancer. 2022 Nov 2;21(1):208. doi: 10.1186/s12943-022-01670-1. Mol Cancer. 2022. PMID: 36324128 Free PMC article. Review.
-
Research Progress of PD 1/PD L1 Inhibitors in the Treatment of Urological Tumors.Curr Cancer Drug Targets. 2024;24(11):1104-1115. doi: 10.2174/0115680096278251240108152600. Curr Cancer Drug Targets. 2024. PMID: 38318829 Review.
References
-
- Mayer F, Stoop H, Scheffer GL, Scheper R, Oosterhuis JW, Looijenga LH, Bokemeyer C. Molecular determinants of treatment response in human germ cell tumors. Clin Cancer Res. 2003;9:767–773. - PubMed
-
- Oechsle K, Honecker F, Cheng T, Mayer F, Czaykowski P, Winquist E, Wood L, Fenner M, Glaesener S, Hartmann JT, et al. Preclinical and clinical activity of sunitinib in patients with cisplatin-refractory or multiply relapsed germ cell tumors: A canadian urologic oncology group/german testicular cancer study group cooperative study. Ann Oncol. 2011;22:2654–2660. doi: 10.1093/annonc/mdr026. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
