Anticancer effects of combinational treatment with BRAFV600E siRNA and PI3K pathway inhibitors in melanoma cell lines harboring BRAFV600E
- PMID: 29928450
- PMCID: PMC6006476
- DOI: 10.3892/ol.2018.8614
Anticancer effects of combinational treatment with BRAFV600E siRNA and PI3K pathway inhibitors in melanoma cell lines harboring BRAFV600E
Abstract
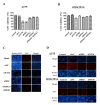
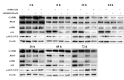
In the present study, the anti-tumor effects of combination treatment with an siRNA targeting B-Raf proto-oncogene serine/threonine kinase (BRAF)V600E and phosphoinositide 3-kinase (PI3K) signaling pathway inhibitors was investigated in melanoma cell lines harboring BRAFV600E. Human melanoma A375 and WM115 cells were treated with siRNA targeting to BRAF or BRAFV600E, combined with treatment with PI3K signaling pathway inhibitors. CCK-8 and EdU proliferation assays were performed to assess cell viability and proliferation, respectively, following treatment. In addition, flow cytometry analysis was performed to determine cell cycle distribution, and western blot analysis was performed to analyze the activity of the extracellular signal-regulated kinase (ERK) and PI3Ksignaling pathways following treatment. Targeting BRAFV600E using small interfering (si)RNA significantly decreased cell viability and DNA replication in tumor cell lines that harbor oncogenic BRAFV600E. Inhibition of BRAFV600E by siRNA combined with treatment with PI3K or mammalian target of rapamycin signaling pathway inhibitors significantly decreased cell viability and proliferation compared with siRNA or inhibitor treatment alone. Concomitant BRAFV600E and PI3K inhibition led to G1/S phase arrest in melanoma cells. However, melanoma cells in which oncogenic BRAFV600E is not highly expressed (WM115 cells) were not sensitive to BRAFV600E targeted therapy. The PI3K signaling pathway inhibitors were more effective in this cell line. The results from the present study provide an insight into the potential effectiveness of combination therapy and personalized cancer treatments.
Keywords: BRAFV600E siRNA; ERK pathway; PI3K pathway; combination therapy; melanoma.
Figures






Similar articles
-
ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells.Mol Cancer. 2017 Jun 8;16(1):102. doi: 10.1186/s12943-017-0667-y. Mol Cancer. 2017. PMID: 28595656 Free PMC article.
-
Computational high-throughput screening and in vitro approaches identify CB-006-3; A novel PI3K-BRAFV600E dual targeted inhibitor against melanoma.Oncol Res. 2022 Oct 10;29(5):305-318. doi: 10.32604/or.2022.025187. eCollection 2021. Oncol Res. 2022. PMID: 37305163 Free PMC article.
-
Conjunctival Melanoma Targeted Therapy: MAPK and PI3K/mTOR Pathways Inhibition.Invest Ophthalmol Vis Sci. 2019 Jun 3;60(7):2764-2772. doi: 10.1167/iovs.18-26508. Invest Ophthalmol Vis Sci. 2019. PMID: 31247083
-
Targeting oncogenic Raf protein-serine/threonine kinases in human cancers.Pharmacol Res. 2018 Sep;135:239-258. doi: 10.1016/j.phrs.2018.08.013. Epub 2018 Aug 15. Pharmacol Res. 2018. PMID: 30118796 Review.
-
Resistance mechanisms in BRAFV600E paediatric high-grade glioma and current therapeutic approaches.Front Oncol. 2022 Dec 13;12:1031378. doi: 10.3389/fonc.2022.1031378. eCollection 2022. Front Oncol. 2022. PMID: 36582791 Free PMC article. Review.
Cited by
-
Surface-Degradable Drug-Eluting Stent with Anticoagulation, Antiproliferation, and Endothelialization Functions.Biomolecules. 2019 Feb 18;9(2):69. doi: 10.3390/biom9020069. Biomolecules. 2019. PMID: 30781704 Free PMC article.
-
Inhibition of Nuclear Pore Complex Formation Selectively Induces Cancer Cell Death.Cancer Discov. 2021 Jan;11(1):176-193. doi: 10.1158/2159-8290.CD-20-0581. Epub 2020 Sep 28. Cancer Discov. 2021. PMID: 32988961 Free PMC article.
-
Peptidomimetics designed to bind to RAS effector domain are promising cancer therapeutic compounds.Sci Rep. 2022 Sep 22;12(1):15810. doi: 10.1038/s41598-022-19703-6. Sci Rep. 2022. PMID: 36138080 Free PMC article.
-
Successes and challenges in modeling heterogeneous BRAFV600E mutated central nervous system neoplasms.Front Oncol. 2023 Oct 18;13:1223199. doi: 10.3389/fonc.2023.1223199. eCollection 2023. Front Oncol. 2023. PMID: 37920169 Free PMC article. Review.
-
Investigating therapeutic efficacy of dacarbazine and temozolomide, alone and in combination with BRAF (V600E) siRNA in A375 human melanoma cell line.Iran J Basic Med Sci. 2025;28(6):772-783. doi: 10.22038/ijbms.2025.84187.18208. Iran J Basic Med Sci. 2025. PMID: 40343301 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
