Aptamers: novelty tools for cancer biology
- PMID: 29928493
- PMCID: PMC6003562
- DOI: 10.18632/oncotarget.25260
Aptamers: novelty tools for cancer biology
Abstract
Although the term 'cancer' was still over two thousand years away of being coined, the first known cases of the disease date back to about 3000BC, in ancient Egypt. Five thousand years later, still lacking a cure, it has become one of the leading causes of death, killing over half a dozen million people yearly. So far, monoclonal antibodies are the most successful immune-therapy tools when it comes to fighting cancer. The number of clinical trials that use them has been increasing steadily during the past few years, especially since the Food and Drug Administration greenlit the use of the first immune-checkpoint blockade antibodies. However, albeit successful, this approach does come with the cost of auto-inflammatory toxicity. Taking this into account, the development of new therapeutic reagents with low toxicity becomes evident, particularly ones acting in tandem with the tools currently at our disposal. Ever since its discovery in the early nineties, aptamer technology has been used for a wide range of diagnostic and therapeutic applications. With similar properties to those of monoclonal antibodies, such as high-specificity of recognition and high-affinity binding, and the advantages of being developed using in vitro selection procedures, aptamers quickly became convenient building blocks for the generation of multifunctional constructs. In this review, we discuss the steps involved in the in vitro selection process that leads to functional aptamers - known as Systematic Evolution of Ligands by Exponential Enrichment - as well as the most recent applications of this technology in diagnostic and treatment of oncological illnesses. Moreover, we also suggest ways to improve such use.
Keywords: Cell-SELEX; aptamers; cancer; clinical application.
Conflict of interest statement
CONFLICTS OF INTEREST There is no conflicts of interest whatsoever regarding this publication.
Figures
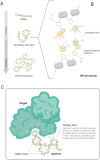
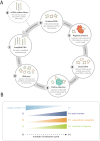
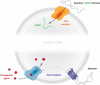
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. https://doi.org/10.3322/caac.20138. - DOI - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. https://doi.org/10.3322/caac.21166. - DOI - PubMed
-
- Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current challenges in cancer treatment. Clin Ther. 2016;38:1551–66. https://doi.org/10.1016/j.clinthera.2016.03.026. - DOI - PubMed
-
- Huang YF, Chang HT, Tan W. Cancer cell targeting using multiple aptamers conjugated on nanorods. Anal Chem. 2008;80:567–72. https://doi.org/10.1021/ac702322j. - DOI - PubMed
-
- Basil CF, Zhao Y, Zavaglia K, Jin P, Panelli MC, Voiculescu S, Mandruzzato S, Lee HM, Seliger B, Freedman RS, Taylor PR, Hu N, Zanovello P, et al. Common cancer biomarkers. Cancer Res. 2006;66:2953–61. https://doi.org/10.1158/0008-5472.CAN-05-3433. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

