A superior bright NIR luminescent nanoparticle preparation and indicating calcium signaling detection in cells and small animals
- PMID: 29928497
- PMCID: PMC5987641
- DOI: 10.1186/s13578-018-0235-1
A superior bright NIR luminescent nanoparticle preparation and indicating calcium signaling detection in cells and small animals
Abstract
Background: Near-field fluorescence (NFF) effects were employed to develop a novel near-infrared (NIR) luminescent nanoparticle (LNP) with superior brightness. The LNP is used as imaging contrast agent for cellular and small animal imaging and furthermore suggested to use for detecting voltage-sensitive calcium in living cells and animals with high sensitivity.
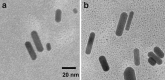
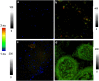
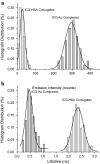
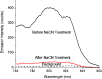
Results: NIR Indocyanine green (ICG) dye was conjugated with human serum albumin (HSA) followed by covalently binding to gold nanorod (AuNR). The AuNR displayed dual plasmons from transverse and longitudinal axis, and the longitudinal plasmon was localized at the NIR region which could efficiently couple with the excitation and emission of ICG dye leading to a largely enhanced NFF. The enhancement factor was measured to be about 16-fold using both ensemble and single nanoparticle spectral methods. As an imaging contrast agent, the ICG-HSA-Au complex (abbreviate as ICG-Au) was conjugated on HeLa cells and fluorescence cell images were recorded on a time-resolved confocal microscope. The emission signals of ICG-Au complexes were distinctly resolved as the individual spots that were observed over the cellular backgrounds due to their strong brightness as well as shortened lifetime. The LNPs were also tested to have a low cytotoxicity. The ICG-Au complexes were injected below the skin surface of mouse showing emission spots 5-fold brighter than those from the same amount of free ICG-HSA conjugates.
Conclusions: Based on the observations in this research, the excitation and emission of NIR ICG dyes were found to be able to sufficiently couple with the longitudinal plasmon of AuNRs leading to a largely enhanced NFF. Using the LNP with super-brightness as a contrast agent, the ICG-Au complex could be resolved from the background in the cell and small animal imaging. The novel NIR LNP has also a great potential for detection of voltage-gated calcium concentration in the cell and living animal with a high sensitivity.
Keywords: Dual-mode plasmons; Fluorescence imaging; Gold nanorod (AUNR); Imaging contrast agent; Indocyanine green (ICG); Luminescent nanoparticle (LNP); Near-field fluorescence (NFF).
Figures



Similar articles
-
Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells.ACS Nano. 2014 Mar 25;8(3):2725-38. doi: 10.1021/nn406425h. Epub 2014 Mar 3. ACS Nano. 2014. PMID: 24571629
-
Intraoperative combined color and fluorescent images-based sentinel node mapping in the porcine lung: comparison of indocyanine green with or without albumin premixing.J Thorac Cardiovasc Surg. 2013 Dec;146(6):1509-15. doi: 10.1016/j.jtcvs.2013.02.044. Epub 2013 Mar 21. J Thorac Cardiovasc Surg. 2013. PMID: 23522603
-
[A new approach for studying the retinal and choroidal circulation].Nippon Ganka Gakkai Zasshi. 2004 Dec;108(12):836-61; discussion 862. Nippon Ganka Gakkai Zasshi. 2004. PMID: 15656089 Review. Japanese.
-
Stabilization of indocyanine green dye in polymeric micelles for NIR-II fluorescence imaging and cancer treatment.Biomater Sci. 2020 Apr 15;8(8):2245-2254. doi: 10.1039/c9bm02010a. Biomater Sci. 2020. PMID: 32129330
-
Bovine serum albumin–stabilized gold nanoclusters conjugated with l-methionine and indocyanine green derivative 02.2012 Oct 1 [updated 2013 Jan 24]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Oct 1 [updated 2013 Jan 24]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23367531 Free Books & Documents. Review.
Cited by
-
Optimizing Calcium Detection Methods in Animal Systems: A Sandbox for Synthetic Biology.Biomolecules. 2021 Feb 24;11(3):343. doi: 10.3390/biom11030343. Biomolecules. 2021. PMID: 33668387 Free PMC article. Review.
-
Optical Detection of Intracellular Quantities Using Nanoscale Technologies.Acc Chem Res. 2019 Jul 16;52(7):1739-1749. doi: 10.1021/acs.accounts.9b00102. Epub 2019 Jun 12. Acc Chem Res. 2019. PMID: 31187980 Free PMC article.
-
Indocyanine green and its nanosynthetic particles for the diagnosis and treatment of hepatocellular carcinoma.Am J Transl Res. 2020 Jun 15;12(6):2344-2352. eCollection 2020. Am J Transl Res. 2020. PMID: 32655776 Free PMC article. Review.
-
Calcium signaling: an underlying link between cardiac disease and carcinogenesis.Cell Biosci. 2018 Jun 8;8:39. doi: 10.1186/s13578-018-0236-0. eCollection 2018. Cell Biosci. 2018. PMID: 29930797 Free PMC article. No abstract available.
References
-
- Burke RC, Bardet SM, Carr L, Romanenko S, Arnaud-Cormos D, Leveque P, O’Connor RP. Nanosecond pulsed electric fields depolarize transmembrane potential via voltage-gated K+, Ca2+ and TRPM8 channels in U87 glioblastoma cells. BBA Biomembr. 2017;1859:2040–2050. doi: 10.1016/j.bbamem.2017.07.004. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

