Endometrial response to conceptus-derived estrogen and interleukin-1β at the time of implantation in pigs
- PMID: 29928500
- PMCID: PMC5989395
- DOI: 10.1186/s40104-018-0259-8
Endometrial response to conceptus-derived estrogen and interleukin-1β at the time of implantation in pigs
Abstract
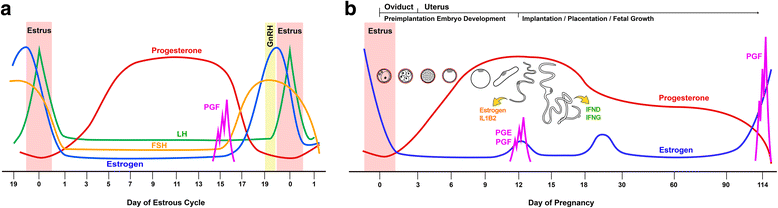
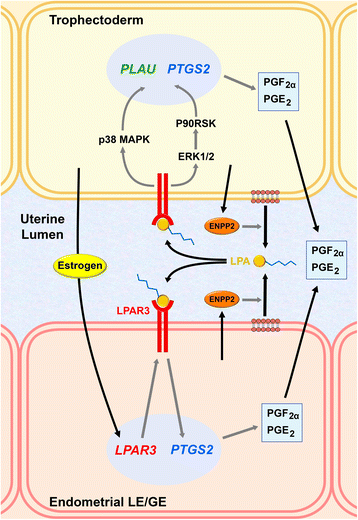
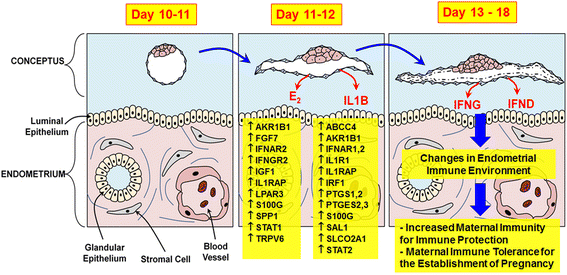
The establishment of pregnancy is a complex process that requires a well-coordinated interaction between the implanting conceptus and the maternal uterus. In pigs, the conceptus undergoes dramatic morphological and functional changes at the time of implantation and introduces various factors, including estrogens and cytokines, interleukin-1β2 (IL1B2), interferon-γ (IFNG), and IFN-δ (IFND), into the uterine lumen. In response to ovarian steroid hormones and conceptus-derived factors, the uterine endometrium becomes receptive to the implanting conceptus by changing its expression of cell adhesion molecules, secretory activity, and immune response. Conceptus-derived estrogens act as a signal for maternal recognition of pregnancy by changing the direction of prostaglandin (PG) F2α from the uterine vasculature to the uterine lumen. Estrogens also induce the expression of many endometrial genes, including genes related to growth factors, the synthesis and transport of PGs, and immunity. IL1B2, a pro-inflammatory cytokine, is produced by the elongating conceptus. The direct effect of IL1B2 on endometrial function is not fully understood. IL1B activates the expression of endometrial genes, including the genes involved in IL1B signaling and PG synthesis and transport. In addition, estrogen or IL1B stimulates endometrial expression of IFN signaling molecules, suggesting that estrogen and IL1B act cooperatively in priming the endometrial function of conceptus-produced IFNG and IFND that, in turn, modulate endometrial immune response during early pregnancy. This review addresses information about maternal-conceptus interactions with respect to endometrial gene expression in response to conceptus-derived factors, focusing on the roles of estrogen and IL1B during early pregnancy in pigs.
Keywords: Conceptus; Endometrium; Estrogen; Interleukin-1β, Pig; Uterus.
Conflict of interest statement
This is a review paper; however, all results reported based on research by the authors was approved by the Institutional Animal Care and Use Committee of Yonsei University.The authors declare that they have no competing interests.
Figures
Similar articles
-
Conceptus-derived cytokines interleukin-1β and interferon-γ induce the expression of acute phase protein serum amyloid A3 in endometrial epithelia at the time of conceptus implantation in pigs.Anim Biosci. 2023 Mar;36(3):441-450. doi: 10.5713/ab.22.0334. Epub 2022 Nov 14. Anim Biosci. 2023. PMID: 36397697 Free PMC article.
-
Transcriptomic analysis of interferon-γ-regulated genes in endometrial explants and their possible role in regulating maternal endometrial immunity during the implantation period in pigs, a true epitheliochorial placentation species.Theriogenology. 2020 Oct 1;155:114-124. doi: 10.1016/j.theriogenology.2020.05.045. Epub 2020 Jun 22. Theriogenology. 2020. PMID: 32659448
-
Implantation and Establishment of Pregnancy in the Pig.Adv Anat Embryol Cell Biol. 2015;216:137-63. doi: 10.1007/978-3-319-15856-3_8. Adv Anat Embryol Cell Biol. 2015. PMID: 26450498 Review.
-
Unique epithelial expression of S100A calcium binding protein A7A in the endometrium at conceptus implantation in pigs.Asian-Australas J Anim Sci. 2019 Feb 9;32(9):1355-1362. doi: 10.5713/ajas.18.0920. Print 2019 Sep. Asian-Australas J Anim Sci. 2019. PMID: 30744322 Free PMC article.
-
Rapid conceptus elongation in the pig: An interleukin 1 beta 2 and estrogen-regulated phenomenon.Mol Reprod Dev. 2017 Sep;84(9):760-774. doi: 10.1002/mrd.22813. Epub 2017 Jun 8. Mol Reprod Dev. 2017. PMID: 28394035 Review.
Cited by
-
Spatial Transcriptomic and miRNA Analyses Revealed Genes Involved in the Mesometrial-Biased Implantation in Pigs.Genes (Basel). 2019 Oct 14;10(10):808. doi: 10.3390/genes10100808. Genes (Basel). 2019. PMID: 31615128 Free PMC article.
-
The Neuropathic Itch Caused by Pseudorabies Virus.Pathogens. 2020 Mar 31;9(4):254. doi: 10.3390/pathogens9040254. Pathogens. 2020. PMID: 32244386 Free PMC article. Review.
-
Can trophectoderm RNA analysis predict human blastocyst competency?Syst Biol Reprod Med. 2019 Aug;65(4):312-325. doi: 10.1080/19396368.2019.1625085. Epub 2019 Jun 27. Syst Biol Reprod Med. 2019. PMID: 31244343 Free PMC article.
-
Expression of circulating oar-miR-485-5p and oar-miR-493-5p during the estrous cycle and early pregnancy in ovine plasma.Anim Reprod. 2024 Mar 4;21(1):e20230115. doi: 10.1590/1984-3143-AR2023-0115. eCollection 2024. Anim Reprod. 2024. PMID: 38510567 Free PMC article.
-
Urinary estrogens as a non-invasive biomarker of viable pregnancy in the giant panda (Ailuropoda melanoleuca).Sci Rep. 2019 Sep 4;9(1):12772. doi: 10.1038/s41598-019-49288-6. Sci Rep. 2019. PMID: 31484972 Free PMC article.
References
-
- Pope WF. Embryonic mortality in swine. In: Zavy MT, Geisert RD, editors. Embryonic Mortality in Domestic Species. Boca Raton: CRC Press; 1994. pp. 53–77.
-
- Mathew DJ, Newsom EM, Guyton JM, Tuggle CK, Geisert RD, Lucy MC. Activation of the transcription factor nuclear factor-kappa B in uterine luminal epithelial cells by interleukin 1 Beta 2: a novel interleukin 1 expressed by the elongating pig conceptus. Biol Reprod. 2015;92(4):107. doi: 10.1095/biolreprod.114.126128. - DOI - PubMed
-
- Jaeger LA, Johnson GA, Ka H, Garlow JG, Burghardt RC, Spencer TE, et al. Functional analysis of autocrine and paracrine signalling at the uterine-conceptus interface in pigs. Reprod Suppl. 2001;58:191–207. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

