Can muscle protein metabolism be specifically targeted by exercise training in COPD?
- PMID: 29928519
- PMCID: PMC5989100
- DOI: 10.21037/jtd.2018.02.67
Can muscle protein metabolism be specifically targeted by exercise training in COPD?
Abstract
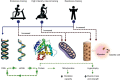
Patients with stable chronic obstructive pulmonary disease (COPD) frequently exhibit unintentional accentuated peripheral muscle loss and dysfunction. Skeletal muscle mass in these patients is a strong independent predictor of morbidity and mortality. Factors including protein anabolism/catabolism imbalance, hypoxia, physical inactivity, inflammation, and oxidative stress are involved in the initiation and progression of muscle wasting in these patients. Exercise training remains the most powerful intervention for reversing, in part, muscle wasting in COPD. Independently of the status of systemic or local muscle inflammation, rehabilitative exercise training induces up-regulation of key factors governing skeletal muscle hypertrophy and regeneration. However, COPD patients presenting similar degrees of lung dysfunction do not respond alike to a given rehabilitative exercise stimulus. In addition, a proportion of patients experience limited clinical outcomes, even when exercise training has been adequately performed. Consistently, several reports provide evidence that the muscles of COPD patients present training-induced myogenic activity limitation as exercise training induces a limited number of differentially expressed genes, which are mostly associated with protein degradation. This review summarises the nature of muscle adaptations induced by exercise training, promoted both by changes in the expression of contractile proteins and their function typically controlled by intracellular signalling and transcriptional responses. Rehabilitative exercise training in COPD patients stimulates skeletal muscle mechanosensitive signalling pathways for protein accretion and its regulation during muscle contraction. Exercise training also induces synthesis of myogenic proteins by which COPD skeletal muscle promotes hypertrophy leading to fusion of myogenic cells to the myofiber. Understanding of the biological mechanisms that regulate exercise training-induced muscle growth and regeneration is necessary for implementing therapeutic strategies specifically targeting myogenesis and hypertrophy in these patients.
Keywords: Chronic obstructive pulmonary disease (COPD); anabolism; exercise; hypertrophy; muscle wasting; myogenesis; protein synthesis.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol 2017;53:128-49. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
