Towards a circuit-level understanding of hippocampal CA1 dysfunction in Alzheimer's disease across anatomical axes
- PMID: 29928558
- PMCID: PMC6005196
Towards a circuit-level understanding of hippocampal CA1 dysfunction in Alzheimer's disease across anatomical axes
Abstract
The hippocampus has been a primary region of study with regards to synaptic and functional changes in Alzheimer’s disease (AD) due to its involvement in early stages, specifically area CA1. However, most work in this area has treated CA1 as a homogeneous structure comprised of uniform neural circuits. Yet, there is a plethora of evidence that CA1 varies in its structure and function across anatomical axes. Here I review the heterogeneity of the functional and circuit architecture of hippocampal area CA1 across three primary anatomical axes. I also summarize evidence that AD differentially affects these subregions, as well as hypotheses as to why this may occur.
Keywords: Alzheimer’s disease; Hippocampus; CA1; entorhinal cortex; Pyramidal neuron.
Figures

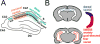


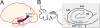
References
-
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. - PubMed
-
- Baimbridge KG, Miller JJ. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982;245:223–229. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
