The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis
- PMID: 29928650
- PMCID: PMC5989754
- DOI: 10.1002/acn3.553
The NRF2 pathway as potential biomarker for dimethyl fumarate treatment in multiple sclerosis
Abstract
Objective: Immunological studies have demonstrated a plethora of beneficial effects of dimethyl fumarate (DMF) on various cell types. However, the cellular and molecular targets are incompletely understood and response markers are scarce. Here, we focus on the relation between nuclear factor (erythroid-derived 2)-like 2 (NRF2) pathway induction under DMF therapy and the composition of the blood immune cell compartment and clinical efficacy in relapsing-remitting multiple sclerosis (MS) patients.
Methods: We explored effects of DMF on peripheral immune cell subsets by flow cytometric and transcriptional analysis of serial blood samples obtained from 43 MS patients during the first year of therapy.
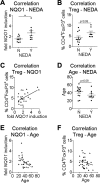
Results: Gene expression analysis proved activation of NRF2 signaling under DMF therapy that was paralleled by a temporal expansion of FoxP3+ regulatory T cells, CD56bright natural killer cells, plasmacytoid dendritic cells, and a decrease in CD8+ T cells, B cells, and type 1 myeloid dendritic cells. In a subgroup of 28 patients with completely available clinical data, individuals with higher levels of the NRF2 target gene NAD(P)H quinone dehydrogenase 1 (NQO1) 4-6 weeks after DMF therapy initiation were more likely to achieve no evidence of disease activity status 1 year later. The degree of NQO1 induction further correlated with patient age.
Interpretation: We demonstrate that positive effects of DMF on the clinical outcome are paralleled by induction of the antioxidant NRF2 transcriptional pathway and a shift toward regulatory immune cell subsets in the periphery. Our data identify a role of the NRF2 pathway as potential biomarker for DMF treatment in MS.
Figures


Similar articles
-
Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the Phase 3 DEFINE and CONFIRM studies.Mult Scler. 2017 Dec;23(14):1875-1883. doi: 10.1177/1352458517690617. Epub 2017 Feb 3. Mult Scler. 2017. PMID: 28156185 Clinical Trial.
-
Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):4777-82. doi: 10.1073/pnas.1603907113. Epub 2016 Apr 13. Proc Natl Acad Sci U S A. 2016. PMID: 27078105 Free PMC article.
-
Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients.Mult Scler. 2016 Jul;22(8):1061-1070. doi: 10.1177/1352458515608961. Epub 2015 Oct 12. Mult Scler. 2016. PMID: 26459150 Free PMC article.
-
Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview.Ther Adv Neurol Disord. 2015 Jan;8(1):20-30. doi: 10.1177/1756285614564152. Ther Adv Neurol Disord. 2015. PMID: 25584071 Free PMC article. Review.
-
Emerging Understanding of the Mechanism of Action for Dimethyl Fumarate in the Treatment of Multiple Sclerosis.Front Neurol. 2018 Jan 23;9:5. doi: 10.3389/fneur.2018.00005. eCollection 2018. Front Neurol. 2018. PMID: 29410647 Free PMC article. Review.
Cited by
-
Alzheimer's Disease Pathogenic Mechanisms: Linking Redox Homeostasis and Mitochondria-Associated Metabolic Pathways Through Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2).Antioxidants (Basel). 2025 Jul 1;14(7):812. doi: 10.3390/antiox14070812. Antioxidants (Basel). 2025. PMID: 40722916 Free PMC article. Review.
-
The lncRNA LUCAT1 is elevated in inflammatory disease and restrains inflammation by regulating the splicing and stability of NR4A2.Proc Natl Acad Sci U S A. 2023 Jan 3;120(1):e2213715120. doi: 10.1073/pnas.2213715120. Epub 2022 Dec 28. Proc Natl Acad Sci U S A. 2023. PMID: 36577072 Free PMC article.
-
Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis.Genes (Basel). 2022 Jul 25;13(8):1324. doi: 10.3390/genes13081324. Genes (Basel). 2022. PMID: 35893061 Free PMC article.
-
Plasma microRNA Signature as Predictive Marker of Clinical Response to Therapy During Multiple Sclerosis.Ann Clin Transl Neurol. 2025 Aug;12(8):1595-1607. doi: 10.1002/acn3.70093. Epub 2025 Jun 11. Ann Clin Transl Neurol. 2025. PMID: 40501038 Free PMC article.
-
Increased NK Cell Count in Multiple Sclerosis Patients Treated With Dimethyl Fumarate: A 2-Year Longitudinal Study.Front Immunol. 2019 Jul 19;10:1666. doi: 10.3389/fimmu.2019.01666. eCollection 2019. Front Immunol. 2019. PMID: 31379857 Free PMC article.
References
-
- Compston A, Coles A. Multiple sclerosis.Lancet Lond Engl 2008;372:1502–1517. - PubMed
-
- Stadelmann C, Brück W. Interplay between mechanisms of damage and repair in multiple sclerosis. J Neurol 2008;255:12–18. - PubMed
-
- Fox RJ, Miller DH, Phillips JT, et al. Placebo‐controlled phase 3 study of oral BG‐12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–1097. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

