Cholecystokinin Receptor-Targeted Polyplex Nanoparticle Inhibits Growth and Metastasis of Pancreatic Cancer
- PMID: 29928669
- PMCID: PMC6008260
- DOI: 10.1016/j.jcmgh.2018.02.013
Cholecystokinin Receptor-Targeted Polyplex Nanoparticle Inhibits Growth and Metastasis of Pancreatic Cancer
Abstract
Background & aims: Pancreatic ductal adenocarcinoma (PDAC) remains the most aggressive malignancy with the lowest 5-year survival rate of all cancers in part owing to the lack of tumor-specific therapy and the rapid metastatic nature of this cancer. The gastrointestinal peptide gastrin is a trophic peptide that stimulates growth of PDAC in an autocrine fashion by interaction with the cholecystokinin receptor that is overexpressed in this malignancy.
Methods: We developed a therapeutic novel polyplex nanoparticle (NP) that selectively targets the cholecystokinin receptor on PDAC. The NP was characterized in vitro and stability testing was performed in human blood. The effects of the target-specific NP loaded with gastrin small interfering RNA (siRNA) was compared with an untargeted NP and with an NP loaded with a scrambled siRNA in vitro and in 2 orthotopic models of PDAC. A polymerase chain reaction metastasis array examined differentially expressed genes from control tumors compared with tumors of mice treated with the targeted polyplex NP.
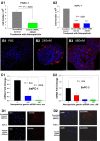
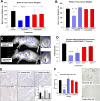
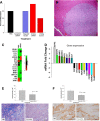
Results: The polyplex NP forms a micelle that safely delivers specific gastrin siRNA to the tumor without off-target toxicity. Consistent with these findings, cellular uptake was confirmed only with the targeted fluorescently labeled NP by confocal microscopy in vitro and by IVIS fluorescent based imaging in mice bearing orthotopic pancreatic cancers but not found with untargeted NPs. Tumor uptake and release of the gastrin siRNA NP was verified by decreased cellular gastrin gene expression by quantitative reverse-transcription polymerase chain reaction and peptide expression by immunohistochemistry. Growth of PDAC was inhibited in a dose-related fashion in cell culture and in vivo. The targeted NP therapy completely blocked tumor metastasis and altered tumor-specific genes.
Conclusions: Our polyplex nanoparticle platform establishes both a strong foundation for the development of receptor-targeted therapeutics and a unique approach for the delivery of siRNA in vivo, thus warranting further exploration of this approach in other types of cancers.
Keywords: CCK Receptor; CCK, cholecystokinin; Ex/Em, maximal excitation and emission wavelengths; Ga-10, gastrin 10 peptide; Gastrin; Gene Therapy; MW, molecular weight; N/P, ratio of “amines” of poly (L-lysine) unit and “phosphates” of siRNA complexed in the polyplex; NMR, nuclear magnetic resonance; NP, nanoparticle; Nanotechnology; Orthotopic; PBS, phosphate-buffered saline; PDAC, pancreatic ductal adenocarcinoma; PEG, polyethylene glycol; PanIN, pancreatic intraepithelial neoplasia; mRNA, messenger RNA; qRT-PCR, quantitative reverse-transcription polymerase chain reaction; siRNA, small interfering RNA.
Figures



Similar articles
-
Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas.Biomolecules. 2021 Nov 25;11(12):1766. doi: 10.3390/biom11121766. Biomolecules. 2021. PMID: 34944412 Free PMC article.
-
Target-Specific Nanoparticle Polyplex Down-Regulates Mutant Kras to Prevent Pancreatic Carcinogenesis and Halt Tumor Progression.Int J Mol Sci. 2023 Jan 1;24(1):752. doi: 10.3390/ijms24010752. Int J Mol Sci. 2023. PMID: 36614194 Free PMC article.
-
Differential expression of gastrin, cholecystokinin-A and cholecystokinin-B receptor mRNA in human pancreatic cancer cell lines.Scand J Gastroenterol. 2001 Jul;36(7):738-43. doi: 10.1080/003655201300192003. Scand J Gastroenterol. 2001. PMID: 11444473
-
Cholecystokinin-B/Gastrin receptor-targeting peptides for staging and therapy of medullary thyroid cancer and other cholecystokinin-B receptor-expressing malignancies.Semin Nucl Med. 2002 Apr;32(2):97-109. doi: 10.1053/snuc.2002.31028. Semin Nucl Med. 2002. PMID: 11965605 Review.
-
The Role of Gastrin and CCK Receptors in Pancreatic Cancer and other Malignancies.Int J Biol Sci. 2016 Jan 28;12(3):283-91. doi: 10.7150/ijbs.14952. eCollection 2016. Int J Biol Sci. 2016. PMID: 26929735 Free PMC article. Review.
Cited by
-
Insights into Effects/Risks of Chronic Hypergastrinemia and Lifelong PPI Treatment in Man Based on Studies of Patients with Zollinger-Ellison Syndrome.Int J Mol Sci. 2019 Oct 16;20(20):5128. doi: 10.3390/ijms20205128. Int J Mol Sci. 2019. PMID: 31623145 Free PMC article. Review.
-
Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications.Life (Basel). 2023 Mar 29;13(4):903. doi: 10.3390/life13040903. Life (Basel). 2023. PMID: 37109432 Free PMC article. Review.
-
Nanoparticles Bounded to Interfering RNAs as a Therapy for Pancreatic Cancer: A Systematic Review.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024 Nov-Dec;16(6):e2013. doi: 10.1002/wnan.2013. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024. PMID: 39510122 Free PMC article.
-
A Theranostic Approach to Target Gastrin in Pancreatic Cancer.Cell Mol Gastroenterol Hepatol. 2018 Apr 24;6(1):117-118.e1. doi: 10.1016/j.jcmgh.2018.04.002. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 29928679 Free PMC article. No abstract available.
-
Cholecystokinin-B Receptor-Targeted Nanoparticle for Imaging and Detection of Precancerous Lesions in the Pancreas.Biomolecules. 2021 Nov 25;11(12):1766. doi: 10.3390/biom11121766. Biomolecules. 2021. PMID: 34944412 Free PMC article.
References
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. - PubMed
-
- Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Ma W.W., Adjei A.A. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

