The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea
- PMID: 29928670
- PMCID: PMC6007821
- DOI: 10.1016/j.jcmgh.2018.02.009
The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea
Abstract
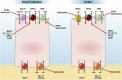
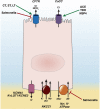
Every year, enteric infections and associated diarrhea kill millions of people. The situation is compounded by increases in the number of enteric pathogens that are acquiring resistance to antibiotics, as well as (hitherto) a relative paucity of information on host molecular targets that may contribute to diarrhea. Many forms of diarrheal disease depend on the dysregulation of intestinal ion transporters, and an associated imbalance between secretory and absorptive functions of the intestinal epithelium. A number of major transporters have been implicated in the pathogenesis of diarrheal diseases and thus an understanding of their expression, localization, and regulation after infection with various bacteria, viruses, and protozoa likely will prove critical in designing new therapies. This article surveys our understanding of transporters that are modulated by specific pathogens and the mechanism(s) involved, thereby illuminating targets that might be exploited for new therapeutic approaches.
Keywords: ATP, adenosine triphosphate; ATPase, adenosine triphosphatase; CDI, Clostridium difficile infection; CFTR, cystic fibrosis transmembrane conductance regulator; CLCA1, chloride channel accessory 1; CT, cholera toxin; CXCR2, C-X-C motif chemokine receptor 2; DRA, down-regulated in adenoma; Diarrhea; ENaC, epithelial sodium channel; EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli; Enteric Pathogen; Epithelium; EspG, Escherichia coli secreted protein G; GPR39, G-protein coupled receptor 39; Ion Transport; KCC, potassium-chloride cotransporter; LPA, lysophosphatidic acid; LT, heat-labile toxin; NHE, sodium/hydrogen exchanger; NHERF2, sodium/hydrogen exchanger regulatory factor 2; NKCC, sodium-potassium-2 chloride cotransporter; ORT, oral rehydration therapy; PKC, protein kinase C; SGLT1, sodium-glucose cotransporter 1; SLC, solute carrier; ST, heat-stabile toxin; TNF, tumor necrosis factor; Tcd, Clostridium difficile toxin; ZnR, zinc sensing receptor; cAMP, adenosine 3′,5′-cyclic monophosphate.
Figures
References
-
- Barrett K.E., Keely S.J. Integrative physiology and pathophysiology of intestinal electrolyte transport. In: Johnson L.R., editor. 4th ed. Vol. 1 and 2. Academic Press; San Diego: 2006. pp. 1931–1951. (Physiology of the Gastrointestinal Tract).
-
- Wright E.M., Loo D.D., Hirayama B.A. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. - PubMed
-
- Schweinfest C.W., Spyropoulos D.D., Henderson K.W., Kim J.H., Chapman J.M., Barone S., Worrell R.T., Wang Z., Soleimani M. slc26a3 (dra)-deficient mice display chloride-losing diarrhea, enhanced colonic proliferation, and distinct up-regulation of ion transporters in the colon. J Biol Chem. 2006;281:37962–37971. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

