Lower Blood Alcohol Concentration Among HIV-Positive Versus HIV-Negative Individuals Following Controlled Alcohol Administration
- PMID: 29928776
- PMCID: PMC6120761
- DOI: 10.1111/acer.13816
Lower Blood Alcohol Concentration Among HIV-Positive Versus HIV-Negative Individuals Following Controlled Alcohol Administration
Abstract
Background: Although it has been purported that HIV-positive individuals may experience a greater degree of intoxication than HIV-negative individuals following acute alcohol consumption, no research to date has empirically tested this supposition. The present investigation entailed a randomized controlled experiment to identify whether the administration of a weight-specified dose of alcohol would lead to differential blood alcohol concentrations (BACs) among HIV-positive versus HIV-negative men.
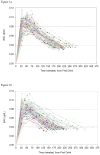
Methods: In a specialized barroom laboratory, 143 men (n = 76 HIV-positive and n = 67 HIV-negative; mean age = 42.9) consumed beverages based on a formulation of 0.7 g alcohol/kg body weight over a 15-minute time frame. BAC was assessed via breathalyzer at 2 set time points (10 and 13 minutes postconsumption) and then periodically until detoxification (BAC < 0.040%). Primary outcomes included (i) area under the curve (AUC), calculated based on all of one's BAC readings, (ii) "BAC-EXP," defined as one's BAC reading 13 minutes postconsumption, and (iii) BAC-PEAK, defined as one's highest recorded BAC reading.
Results: Contrary to predictions, AUC (t(141) = 2.23, p = 0.027), BAC-EXP (t(141) = 2.68, p = 0.008), and BAC-PEAK (t(141) = 2.29, p = 0.023) were significantly lower among HIV-positive versus HIV-negative participants. These effects were sustained in multivariable models controlling for age, race, and AUDIT-based hazardous drinking classification. Among the HIV-positive sample, outcomes did not significantly differ based on HIV viral load detectability, antiretroviral therapy (ART) status, or ART adherence.
Conclusions: The administration of a controlled, weight-specified dose of alcohol led to lower BACs among HIV-positive versus HIV-negative participants. These differences might derive from decreased body fat percentage and delayed gastric emptying associated with HIV seropositivity; however, additional research is necessary to verify these mechanisms. Unique alcohol dosing formulas based on HIV serostatus may be required in future alcohol administration experiments involving HIV-positive samples.
Keywords: HIV; Alcohol; Blood Alcohol Concentration; Experiment; Intoxication.
Copyright © 2018 by the Research Society on Alcoholism.
Figures
Similar articles
-
Drivers who self-estimate lower blood alcohol concentrations are riskier drivers after drinking.Psychopharmacology (Berl). 2016 Apr;233(8):1387-94. doi: 10.1007/s00213-016-4233-x. Epub 2016 Feb 10. Psychopharmacology (Berl). 2016. PMID: 26861796 Free PMC article.
-
Road safety implications of the blood alcohol concentrations among alcohol users exiting bars in northern Ghana.Traffic Inj Prev. 2018;19(8):799-805. doi: 10.1080/15389588.2018.1503415. Epub 2019 Jan 25. Traffic Inj Prev. 2018. PMID: 30681893
-
Effect of a Snack Bar Optimized to Reduce Alcohol Bioavailability: A Randomized Controlled Clinical Trial in Healthy Individuals.J Med Food. 2020 Apr;23(4):432-439. doi: 10.1089/jmf.2019.0228. Epub 2019 Nov 22. J Med Food. 2020. PMID: 31755823 Free PMC article. Clinical Trial.
-
Validity of self-reported alcohol consumption in nondependent drinkers with unintentional injuries.Alcohol Clin Exp Res. 2000 Sep;24(9):1406-13. Alcohol Clin Exp Res. 2000. PMID: 11003207 Clinical Trial.
-
Best-practices approach to determination of blood alcohol concentration (BAC) at specific time points: Combination of ante-mortem alcohol pharmacokinetic modeling and post-mortem alcohol generation and transport considerations.Regul Toxicol Pharmacol. 2016 Jul;78:24-36. doi: 10.1016/j.yrtph.2016.03.020. Epub 2016 Apr 1. Regul Toxicol Pharmacol. 2016. PMID: 27041394 Review.
Cited by
-
Factors associated with phosphatidylethanol (PEth) sensitivity for detecting unhealthy alcohol use: An individual patient data meta-analysis.Alcohol Clin Exp Res. 2021 Jun;45(6):1166-1187. doi: 10.1111/acer.14611. Epub 2021 May 7. Alcohol Clin Exp Res. 2021. PMID: 33837975 Free PMC article.
-
Correlates of high phosphatidylethanol (PEth) levels and their concordance with self-reported heavy alcohol consumption among men who have sex with men who binge drink alcohol.Alcohol Clin Exp Res. 2022 Aug;46(8):1565-1579. doi: 10.1111/acer.14891. Epub 2022 Jul 8. Alcohol Clin Exp Res. 2022. PMID: 35722862 Free PMC article. Clinical Trial.
-
Spatial Analysis of HIV Determinants Among Females Aged 15-34 in KwaZulu Natal, South Africa: A Bayesian Spatial Logistic Regression Model.Int J Environ Res Public Health. 2025 Mar 17;22(3):446. doi: 10.3390/ijerph22030446. Int J Environ Res Public Health. 2025. PMID: 40238564 Free PMC article.
-
A conceptual model of alcohol use and adherence to antiretroviral therapy: systematic review and theoretical implications for mechanisms of action.Health Psychol Rev. 2022 Mar;16(1):104-133. doi: 10.1080/17437199.2020.1806722. Epub 2020 Aug 20. Health Psychol Rev. 2022. PMID: 32757813 Free PMC article.
-
Change in Alcohol Use Based on Self-Report and a Quantitative Biomarker, Phosphatidylethanol, in People With HIV.AIDS Behav. 2022 Mar;26(3):786-794. doi: 10.1007/s10461-021-03438-y. Epub 2021 Sep 20. AIDS Behav. 2022. PMID: 34542779
References
-
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG World Health Organization. Dept. of Mental Health and Substance Dependence. The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. 2. World Health Organization; Geneva: 2001.
-
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical