Altered synaptic and firing properties of cerebellar Purkinje cells in a mouse model of ARSACS
- PMID: 29928778
- PMCID: PMC6117548
- DOI: 10.1113/JP275902
Altered synaptic and firing properties of cerebellar Purkinje cells in a mouse model of ARSACS
Erratum in
-
Corrigendum.J Physiol. 2019 Jul;597(13):3503. doi: 10.1113/JP278333. Epub 2019 Jun 6. J Physiol. 2019. PMID: 31257620 No abstract available.
Abstract
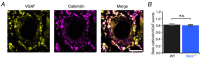
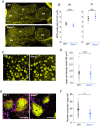
Key points: Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is an early-onset neurodegenerative human disease characterized in part by ataxia and Purkinje cell loss in anterior cerebellar lobules. A knock-out mouse model has been developed that recapitulates several features of ARSACS. Using this ARSACS mouse model, we report changes in synaptic input and intrinsic firing in cerebellar Purkinje cells, as well as in their synaptic output in the deep cerebellar nuclei. Changes in firing are observed in anterior lobules that later exhibit Purkinje cell death, but not in posterior lobules that do not. Our results show that both synaptic and intrinsic alterations in Purkinje cell properties likely contribute to disease manifestation in ARSACS; these findings resemble pathophysiological changes reported in several other ataxias.
Abstract: Autosomal recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) is an early-onset neurodegenerative disease that includes a pronounced and progressive cerebellar dysfunction. ARSACS is caused by an autosomal recessive loss-of-function mutation in the Sacs gene that encodes the protein sacsin. To better understand the cerebellar pathophysiology in ARSACS, we studied synaptic and firing properties of Purkinje cells from a mouse model of ARSACS, Sacs-/- mice. We found that excitatory synaptic drive was reduced onto Sacs-/- Purkinje cells, and that Purkinje cell firing rate, but not regularity, was reduced at postnatal day (P)40, an age when ataxia symptoms were first reported. Firing rate deficits were limited to anterior lobules that later display Purkinje cell death, and were not observed in posterior lobules where Purkinje cells are not lost. Mild firing deficits were observed as early as P20, prior to the manifestation of motor deficits, suggesting that a critical level of cerebellar dysfunction is required for motor coordination to emerge. Finally, we observed a reduction in Purkinje cell innervation onto target neurons in the deep cerebellar nuclei (DCN) in Sacs-/- mice. Together, these findings suggest that multiple alterations in the cerebellar circuit including Purkinje cell input and output contribute to cerebellar-related disease onset in ARSACS.
Keywords: action potential; cerebellum; deep cerebellar nuclei; synaptic currents.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures





References
-
- Bouchard JP, Barbeau A, Bouchard R & Bouchard RW (1978). Autosomal recessive spastic ataxia of Charlevoix‐Saguenay. Can J Neurol Sci 5, 61–69. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

