Partial Tmem106b reduction does not correct abnormalities due to progranulin haploinsufficiency
- PMID: 29929528
- PMCID: PMC6013889
- DOI: 10.1186/s13024-018-0264-6
Partial Tmem106b reduction does not correct abnormalities due to progranulin haploinsufficiency
Abstract
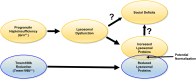
Background: Loss of function mutations in progranulin (GRN) are a major cause of frontotemporal dementia (FTD). Progranulin is a secreted glycoprotein that localizes to lysosomes and is critical for proper lysosomal function. Heterozygous GRN mutation carriers develop FTD with TDP-43 pathology and exhibit signs of lysosomal dysfunction in the brain, with increased levels of lysosomal proteins and lipofuscin accumulation. Homozygous GRN mutation carriers develop neuronal ceroid lipofuscinosis (NCL), an earlier-onset lysosomal storage disorder caused by severe lysosomal dysfunction. Multiple genome-wide association studies have shown that risk of FTD in GRN mutation carriers is modified by polymorphisms in TMEM106B, which encodes a lysosomal membrane protein. Risk alleles of TMEM106B may increase TMEM106B levels through a variety of mechanisms. Brains from FTD patients with GRN mutations exhibit increased TMEM106B expression, and protective TMEM106B polymorphisms are associated with decreased TMEM106B expression. Together, these data raise the possibility that reduction of TMEM106B levels may protect against the pathogenic effects of progranulin haploinsufficiency.
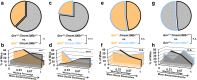
Methods: We crossed Tmem106b +/- mice with Grn +/- mice, which model the progranulin haploinsufficiency of GRN mutation carriers and develop age-dependent social deficits and lysosomal abnormalities in the brain. We tested whether partial Tmem106b reduction could normalize the social deficits and lysosomal abnormalities of Grn +/- mice.
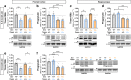
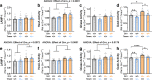
Results: Partial reduction of Tmem106b levels did not correct the social deficits of Grn +/- mice. Tmem106b reduction also failed to normalize most lysosomal abnormalities of Grn +/- mice, except for β-glucuronidase activity, which was suppressed by Tmem106b reduction and increased by progranulin insufficiency.
Conclusions: These data do not support the hypothesis that Tmem106b reduction protects against the pathogenic effects of progranulin haploinsufficiency, but do show that Tmem106b reduction normalizes some lysosomal phenotypes in Grn +/- mice.
Keywords: Frontotemporal dementia; Lysosome; Progranulin; TMEM106B.
Conflict of interest statement
Ethics approval and consent to participate
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

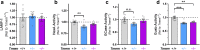
References
-
- Eriksen JL, Mackenzie IR. Progranulin: normal function and role in neurodegeneration. J Neurochem. 2008;104:287–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

