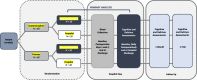
Dexmedetomidine and intravenous acetaminophen for the prevention of postoperative delirium following cardiac surgery (DEXACET trial): protocol for a prospective randomized controlled trial
- PMID: 29929533
- PMCID: PMC6013954
- DOI: 10.1186/s13063-018-2718-0
Dexmedetomidine and intravenous acetaminophen for the prevention of postoperative delirium following cardiac surgery (DEXACET trial): protocol for a prospective randomized controlled trial
Abstract
Background: Postoperative delirium is common in elderly cardiac surgery patients. It is multifactorial and is influenced by the patient's baseline status and the nature of the medical and surgical interventions that the patient receives. Some of these factors are potentially modifiable, including postoperative sedation and analgesia protocols. This study has been designed to evaluate the effectiveness of postoperative intravenous acetaminophen in conjunction with either dexmedetomidine or propofol in decreasing the incidence of delirium.
Methods: This is a prospective, randomized, placebo-controlled, double-blinded, factorial trial that includes patients who are at least 60 years old and who are undergoing cardiac surgeries involving cardiopulmonary bypass, including coronary artery bypass graft (CABG) and combined CABG/valve surgeries. Patients are randomly assigned to receive one of four postoperative analgesic-sedation regimens: (1) acetaminophen and dexmedetomidine, (2) acetaminophen and propofol, (3) dexmedetomidine and placebo, or (4) propofol and placebo. The primary outcome, incidence of delirium, will be assessed with the Confusion Assessment Method (CAM or CAM-ICU). The secondary outcome, postoperative cognitive decline, will be assessed with the Montreal Cognitive Assessment. Additional secondary outcomes, including duration of delirium, postoperative analgesic requirement, length of stay, and incidence of adverse events, will also be reported. Data will be analyzed in 120 randomly assigned patients who received at least one dose of the study medication(s) on a modified intention-to-treat basis.
Discussion: This study has been approved by the institutional review board at Beth Israel Deaconess Medical Center, and the trial is currently recruiting. This study will systematically examine the implications of modification in postoperative sedative/analgesic protocols after cardiac surgery, specifically for short- and long-term cognitive outcomes. Any positive outcomes from this study could direct simple yet effective practice changes aimed to reduce morbidity.
Trial registration: ClinicalTrials.gov Identifier: NCT02546765 , registered January 13, 2015.
Keywords: Acetaminophen; Cardiac surgery; Confusion Assessment Method; Coronary artery bypass grafting; Dexmedetomidine; Montreal Cognitive Assessment; Neurocognition; Postoperative delirium; Propofol.
Conflict of interest statement
Ethics approval and consent to participate
This study has been approved by the Committee on Clinical Investigations Institutional Review Board at Beth Israel Deaconess Medical Center (Protocol 2014-P-000413). Written informed consent will be obtained from all subjects prior to initiation of study procedures by a trained study staff member. This will include discussion about all the aspects of the study, including drug intervention, assessments, follow-up, and acquisition and storage of blood samples.
Consent for publication
Not applicable.
Competing interests
The principal investigator is supported by National Institutes of Health Research Project Grant GM 098406. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Effect of Intravenous Acetaminophen vs Placebo Combined With Propofol or Dexmedetomidine on Postoperative Delirium Among Older Patients Following Cardiac Surgery: The DEXACET Randomized Clinical Trial.JAMA. 2019 Feb 19;321(7):686-696. doi: 10.1001/jama.2019.0234. JAMA. 2019. PMID: 30778597 Free PMC article. Clinical Trial.
-
Comparison of dexmedetomidine versus propofol for sedation in mechanically ventilated patients after cardiovascular surgery.Methodist Debakey Cardiovasc J. 2014 Apr-Jun;10(2):111-7. doi: 10.14797/mdcj-10-2-111. Methodist Debakey Cardiovasc J. 2014. PMID: 25114763 Free PMC article.
-
Propofol plus low-dose dexmedetomidine infusion and postoperative delirium in older patients undergoing cardiac surgery.Br J Anaesth. 2021 Mar;126(3):665-673. doi: 10.1016/j.bja.2020.10.041. Epub 2020 Dec 24. Br J Anaesth. 2021. PMID: 33358336 Clinical Trial.
-
Effectiveness of dexmedetomidine versus propofol on extubation times, length of stay and mortality rates in adult cardiac surgery patients: a systematic review and meta-analysis.JBI Database System Rev Implement Rep. 2018 May;16(5):1220-1239. doi: 10.11124/JBISRIR-2017-003488. JBI Database System Rev Implement Rep. 2018. PMID: 29762314
-
Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials.J Crit Care. 2017 Apr;38:190-196. doi: 10.1016/j.jcrc.2016.10.026. Epub 2016 Nov 11. J Crit Care. 2017. PMID: 27936404 Review.
Cited by
-
Pharmacological strategies to prevent postoperative delirium: a systematic review and network meta-analysis.Anesth Pain Med (Seoul). 2021 Jan;16(1):28-48. doi: 10.17085/apm.20079. Epub 2021 Jan 15. Anesth Pain Med (Seoul). 2021. PMID: 33445233 Free PMC article.
-
Related Factors and Treatment of Postoperative Delirium in Old Adult Patients: An Integrative Review.Healthcare (Basel). 2021 Aug 26;9(9):1103. doi: 10.3390/healthcare9091103. Healthcare (Basel). 2021. PMID: 34574877 Free PMC article. Review.
-
Twelve-Month Cognitive and Functional Outcomes Following Cardiac Surgery: The DEXACET Trial of Intravenous Acetaminophen Versus Placebo.Front Pharmacol. 2022 Mar 22;13:803903. doi: 10.3389/fphar.2022.803903. eCollection 2022. Front Pharmacol. 2022. PMID: 35392551 Free PMC article.
-
Intravenous acetaminophen for postoperative delirium in older patients recovering from major non-cardiac surgery: a randomised-controlled study protocol.BMJ Open. 2025 May 15;15(5):e097079. doi: 10.1136/bmjopen-2024-097079. BMJ Open. 2025. PMID: 40379334 Free PMC article.
-
The effect of thoracic epidural analgesia on short-term outcome and mortality in geriatric patients undergoing open heart surgery.Ulus Travma Acil Cerrahi Derg. 2022 Mar;28(3):382-389. doi: 10.14744/tjtes.2022.57995. Ulus Travma Acil Cerrahi Derg. 2022. PMID: 35485565 Free PMC article.
References
-
- Bellelli G, Morandi A, Di Santo SG, Mazzone A, Cherubini A, Mossello E, Bo M, Bianchetti A, Rozzini R, Zanetti E, et al. “Delirium Day”: a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016;14(1):106. doi: 10.1186/s12916-016-0649-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical