The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics
- PMID: 29929556
- PMCID: PMC6013893
- DOI: 10.1186/s12931-018-0784-1
The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics
Abstract
The Human Viral Challenge (HVC) model has, for many decades, helped in the understanding of respiratory viruses and their role in disease pathogenesis. In a controlled setting using small numbers of volunteers removed from community exposure to other infections, this experimental model enables proof of concept work to be undertaken on novel therapeutics, including vaccines, immunomodulators and antivirals, as well as new diagnostics.Crucially, unlike conventional phase 1 studies, challenge studies include evaluable efficacy endpoints that then guide decisions on how to optimise subsequent field studies, as recommended by the FDA and thus licensing studies that follow. Such a strategy optimises the benefit of the studies and identifies possible threats early on, minimising the risk to subsequent volunteers but also maximising the benefit of scarce resources available to the research group investing in the research. Inspired by the principles of the 3Rs (Replacement, Reduction and Refinement) now commonly applied in the preclinical phase, HVC studies allow refinement and reduction of the subsequent development phase, accelerating progress towards further statistically powered phase 2b studies. The breadth of data generated from challenge studies allows for exploration of a wide range of variables and endpoints that can then be taken through to pivotal phase 3 studies.We describe the disease burden for acute respiratory viral infections for which current conventional development strategies have failed to produce therapeutics that meet clinical need. The Authors describe the HVC model's utility in increasing scientific understanding and in progressing promising therapeutics through development.The contribution of the model to the elucidation of the virus-host interaction, both regarding viral pathogenicity and the body's immunological response is discussed, along with its utility to assist in the development of novel diagnostics.Future applications of the model are also explored.
Keywords: Acute respiratory infections; Respiratory medicine; Controlled clinical trial; Diagnostic; Flu; Gene switching; Human boca virus (HBoV). Therapeutics; Human rhinovirus (HRV); Immune response; Pathogenicity; Pragmatic clinical trials; Research and development; Respiratory syncytial virus (RSV); Virus-host interactions; influenza.
Conflict of interest statement
Ethics approval and consent to participate
All clinical studies were described received appropriate Ethical Committee approval, including informed consent of volunteers.
Competing interests
All authors declare that they are employees of
(ISMPP) ‘Good Publication Practice for Communicating Company-Sponsored Medical Research: GPP3’.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
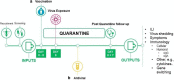
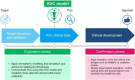
References
-
- Jamison DT, World Bank., Disease Control Priorities Project . Disease control priorities in developing countries. 2. New York Washington, DC: Oxford University Press ;World Bank; 2006.
-
- Excess Winter Mortality in England and Wales : 2014/15 (Provisional) and 2013/14 (Final) [https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarri...].
-
- Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of mortality attributable to influenza and RSV in the United States during 1997-2009 by influenza type or subtype, age, cause of death, and risk status. Influenza Other Respir Viruses. 2014;8:507–515. doi: 10.1111/irv.12258. - DOI - PMC - PubMed
-
- Pinzon-Rondon AM, Aguilera-Otalvaro P, Zarate-Ardila C, Hoyos-Martinez A. Acute respiratory infection in children from developing nations: a multi-level study. Paediatr Int Child Health. 2016:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

